Long Answer Type
Long Answer Type(a) What is fragmentation in organisms? Name a multicellular organism which reproduces by this method.
(b) What is regeneration in organism? Describe regeneration in Planaria with the help of a suitable diagram.
(a) What is fragmentation in organisms? Name a multicellular organism which reproduces by this method.
(b) What is regeneration in an organism? Describe regeneration in Planaria with the help of a suitable diagram.
(a) In a tabular form, differentiate between ethanol and ethanoic acid under the following heads:
(i) Physical state
(ii) Taste
(iii) NaHCO3 test
(iv) Ester test
(b) Write a chemical reaction to show the dehydration of ethanol.
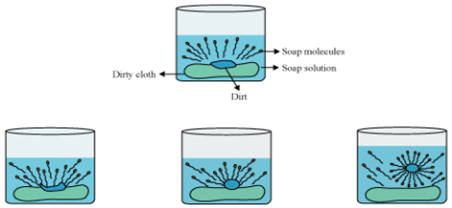
(a) What is soap? Why are soaps not suitable for washing clothes when the water is hard?
(b) Explain the action of soap in removing an oily spot from a piece of cloth.
(a) Soap is sodium or potassium salt of higher fatty acids such as oleic acid (C17H33COOH), stearic acid (C17H35COOH), palimitic acid (C15H31COOH), etc.
A soap is a sodium or potassium salt of long chain fatty acids. Hard water contains salts of calcium and magnesium. On adding soap to water, calcium and magnesium ions present in water displace sodium or potassium ions from the soap molecules forming an insoluble substance called scum. Scum results in wastage of soap.
(b) Cleansing action of soaps:
The oily spot present on clothes is organic in nature and insoluble in water. Therefore, it cannot be removed by only washing with water. When soap is dissolved in water, its hydrophobic ends attach themselves to the oily spot and remove it from the cloth. Then, the molecules of soap arrange themselves in the form of micelle and trap the dirt at the centre of the cluster. These micelles remain suspended in the water. Hence, the oily spots are easily rinsed away by water.