 Short Answer Type
Short Answer TypeWhat does HIV stand for? Is AIDS an infectious disease? List any four modes of spreading AIDS.
Describe any three ways in which individuals with a particular trait may increase in population.
 Long Answer Type
Long Answer Typea) State two properties of carbon which lead to a very large number of carbon compounds.
b) Why does micelle formation take place when soap is added to water? why are micelles not formed when soap is added to ethanol ?
Or
Explain isomerism. State any four characteristics of isomers. Draw the structure of possible isomers of butane C4H10.
a) 1. Catenation: Catenation is the unique property of carbon atoms to form bonds with other atoms of carbon giving rise to large molecules.
2. Tetravalency: Carbon has a four valency. It capable of bonding with four other atoms of carbon atom or other some monovalent elements.
b) The soap molecule is generally represented as RCOONa. In solution, it ionises to form RCOO- and Na+. Each soap molecule has a polar head group (carboxylate ion, COO- group) and a long non-polar hydrocarbon tail (R group from long chain fatty acid). The polar head attracts the polar water molecule and is called hydrophilic end and the non-polar tail attracts the water-insoluble oily or greasy dirt particles.
When a dirty cloth is placed in a soap solution, the long non-polar hydrocarbon tail of soap molecules points towards the oily dirt particles and the polar heads point towards the water. This forms a spherical structure with polar parts of the molecule on the surface and non-polar parts in the centre. This spherical structure is called micelle. This micelle is attracted towards the water and carries the oily dirt particles along with it. This causes the dirt particles to detach from the fibres of the cloth. In this manner, clothes become free from dirt or dust.
Or
Isomerism can be defined as the compound which has same molecular formula but different structural formulae are called isomers and this phenomenon is known as isomerism.
Characteristics of Isomers
i) They have same molecular formula
ii)they have a different structural formula
iii) They differ in physical properties
iv) They have same similar chemical properties.
The structure of possible isomers of butane C4H10.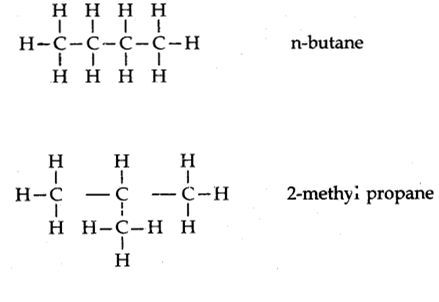
a) What is meant by the 'power of a lens'?
b) State and define the S.I. unit of power of a lens.
c) A convex lens of focal length 25 cm and a concave lens of focal length 10 cm are placed in close contact with each other.
Calculate the lens power of this combination.
What is binary fission in organisms? With the help of suitable diagrams, describe the mode of reproduction in Amoeba.
