 Long Answer Type
Long Answer TypeState the law of refraction of light that defines the refractive index of a medium with respect to the other. Express it mathematically. How is refractive index of any medium 'A' with respect to a medium 'B' related to the speed of propagation of light in two media A and B? State the name of this constant when one medium is vacuum or air.
The refractive indices of glass and water with respect to vacuum are 3/2 and 4/3 respectively. If the speed of light in glass is 2 × m/s, find the speed of light in (i) vacuum, (ii) water.
What is the difference between the chemical composition of soaps and detergents? State in brief the action of soaps in removing an oily spot from a shirt. Why soaps are not considered suitable for washing where water is hard?
Sodium or potassium salts of long chain carboxylic acid are soaps. Whereas, detergents are ammonium or sulphonate salts of long chain carboxylic acid.
Cleansing action of soap:
Dirt which is present on the clothes cannot be removed solely by water as they are inorganic in nature and insoluble in water. When soap is dissolved in water, its hydrophobic ends attach themselves to the dirt and remove it from the cloth. Then, the molecules of soap arrange themselves in micelle formation and trap the dirt at the centre of the cluster. These micelles remain suspended in the water. Hence, the dust particles are easily rinsed away by water.
The diagram details us of the cleansing action of soaps.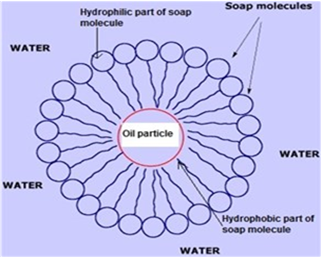
Soaps are not considered suitable when water is hard. Hard water contains salts of calcium and magnesium. A soap is a sodium or potassium salt of long chain fatty acids. When soap is added to hard water, calcium and magnesium ions present in water displace sodium or potassium ions from the soap molecules forming an insoluble substance called scum. The formation of scum results in wastage of an enormous quantity of soap.
List in tabular form three physical and two chemical properties on the basis of which ethanol and ethanoic acid can be differentiated.
