 Short Answer Type
Short Answer TypeList two tests for experimentally distinguishing between an alcohol and a carboxylic acid and describe how these tests are performed.
Draw the electron-dot structure for ethyne. A mixture of ethyne and oxygen is burnt for welding. In your opinion, why cannot we use a mixture of ethyne and air for this purpose ?
Two elements ‘P’ and ‘Q’ belong to the same period of the modern periodic table and are
in Group 1 and Group 2, respectively. Compare their following characteristics in tabular form:
(a) The number of electrons in their atoms
(b) The sizes of their atoms
(c) Their metallic character
(d) Their tendencies to lose electrons
(e) The formula of their oxides
(f) The formula of their chlorides
Taking the example of an element of atomic number 16, explain how the electronic configuration of the atom of an element relates to its position in the modern periodic table and how valency of an element is calculated on the basis of its atomic number.
Both soap and detergent are some type of salts. What is the difference between them? Describe in brief the cleansing action of soap. Why do soaps not form lather in hard water? List two problems that arise due to the use of detergents instead of soaps.
The molecules of soap are sodium or potassium salts of long-chain carboxylic acids. Detergents are generally ammonium or sulphonate salts of long chain carboxylic acids.
Cleansing action of soap can be described as follows:
The soap molecule is generally represented as RCOONa. In solution, it ionises to form RCOO- and Na+. Each soap molecule has a polar head group (carboxylate ion, COO- group) and a long non-polar hydrocarbon tail (R group from long chain fatty acid). The polar head attracts the polar water molecule and is called hydrophilic end and the non-polar tail attracts the water-insoluble oily or greasy dirt particles.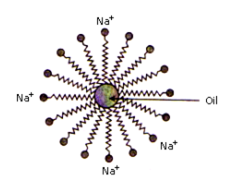
Soaps do not form a lather in hard water because hard water contains calcium and magnesium salts. Soap molecules react with calcium and magnesium salts to form an insoluble precipitate called scum.
Two problems arise because of the use of detergents instead of soap:
i. Soaps are biodegradable, while detergents are non-biodegradable; hence, detergents accumulate in the environment and cause problems.
ii. Certain phosphate additives are added to detergents. These phosphate additives act as nutrients for algae which form a thick green scum over the river water and upset the animal life in the river.
When you add sodium hydrogen carbonate to acetic acid in a test tube, a gas liberates immediately with brisk effervescence. Name this gas. Describe the method of testing this gas.
The absolute refractive indices of glass and water are 4/3 and 3/2, respectively. If the speed of light in glass is 2 × 108m/s, calculate the speed of light in,
a) vacuum
b) water
What is meant by scattering of light? Use this phenomenon to explain why the clear sky appears blue or the Sun appears reddish at sunrise?
