 Short Answer Type
Short Answer TypeExplain how you can determine the atomic mass of an unknown metal if you know its mass density and the dimensions of a unit cell of its crystal.
Differentiate between molarity and molality values for a solution. What is the effect of change in temperature on molarity and molality values?
The thermal decomposition of HCO2H is a first-order reaction with a rate constant of 2.4 x 10-3┬Ās-1┬Āat a certain temperature. Calculate how long will it take for three-fourths of the initial quantity of HCO2H to decompose.
(log 0.25 = - 0.6021)
What do you understand by the rate law and rate constant of a reaction?
Identify the order of a reaction if the units of its rate constant are:
(i)┬ĀL-1┬Āmol s-1
(ii)┬ĀL mol-1┬Ās-1Describe the principle controlling each of the following processes:
(i)┬ĀPreparation of cast iron form pig iron.
(ii)┬ĀPreparation of pure alumina (Al2O3) from bauxite ore.(i)┬ĀThe iron obtained from blast furnaces is known as pig iron. It contains around 4%carbon and many impurities such as S, P, Si, and Mn in smaller amounts.
┬ĀCast iron is obtained by melting pig iron and coke using a hot air blast. It contains a lower amount of carbon (3%) than pig iron; cast iron is extremely hard and brittle.
(ii)┬ĀBauxite usually contains silica, iron oxide, and titanium oxide as impurities. In the process of leaching, alumina is concentrated by digesting the powdered ore with a concentrated solution of NaOH at 473-523 K and 35-36 bar. Under these conditions, alumina (Al2O3) dissolves as sodium meta-aluminate and silica (SiO2) dissolves as sodium silicate leaving the impurities behind.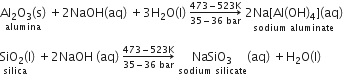
The impurities are then filtered and the solution is neutralized by passing CO2┬Āgas. In this process, hydrated Al2O3┬Āgets precipitated and sodium silicate remains in the solution. Precipitation is induced by seeding the solution with freshly prepared samples of hydrated Al2O3.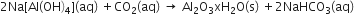
Hydrated alumina thus obtained is filtered, dried, and heated to give back pure alumina (Al2O3).
