 Short Answer Type
Short Answer TypeExplain giving reasons:
(i) Transition metals and their compounds generally exhibit a paramagnetic behaviour.
(ii) The chemistry of actinoids is not as smooth as that of lanthanoids.Complete the following chemical equations: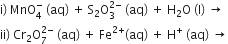
Or
State reasons for the following:
(i) Cu (I) ion is not stable in an aqueous solution.
(ii) Unlike Cr3+, Mn2+, Fe3+ and the subsequent other M2+ ions of the 3d series of elements, the 4d and the 5d series metals generally do not form stable cationic species.
A solution prepared by dissolving 8.95 mg of a gene fragment in 35.0 mL of water has an osmotic pressure of 0.335 torr at 25°C.
Assuming that the gene fragment is a non-electrolyte, calculate its molar mass.
Classify colloids where the dispersion medium is water. State their characteristics and write an example of each of these classes.
OR
Explain what is observed when
(i) An electric current is passed through a sol
(ii) A beam of light is passed through a sol
(iii) An electrolyte (say NaCl) is added to ferric hydroxide solColloids containing dispersion medium as water can be classified as follows:
|
Dispersed phase |
Dispersion medium |
Type of colloid |
Example |
|
Solid |
Liquid |
Sol |
Paints, cell fluids |
|
Liquid |
Liquid |
Emulsion |
Milk, hair cream |
|
Gas |
Liquid |
Foam |
Froth, soap lather |
OR
(i) The colloidal particles are charged and carry either a positive or a negative charge. The dispersion medium carries an equal and opposite charge. This makes the entire system neutral. Under the influence of an electric current, the colloidal particles move towards the oppositely charged electrode. When they come in contact with the electrode, they lose their charge and coagulate.
(ii) When a beam of light is passed through a colloidal solution, a scattering of light is observed. This is known as Tyndall effect. This scattering of light illuminates the path of the beam in the colloidal solution.
(iii) When NaCl is added to hydrated ferric hydroxide sol, it dissociates to give Na+ and Cl- ions. Particles of ferric hydroxide sol are positively charged. This neutralises the colloidal particles which then unite to form bigger particles that are consequently precipitated. Thus, they get coagulated in the presence of negatively charged Cl- ions.
How would you account for the following?
(i) NF3 is an exothermic compound but NCl3 is not.
(ii) The acidic strength of compounds increases in the order:
PH3 < H2S < HCl
(iii) SF6 is kinetically inert.Write the state of hybridization, the shape and the magnetic behaviour of the following complex entities:
(i) [Cr (NH3)4 Cl2] Cl
(ii) [Co (en) 3] Cl3
(iii) K2 [Ni (CN) 4]