Short Answer Type
Short Answer TypeWrite the main structural difference between DNA and RNA. Of the four bases, name those which are common to both DNA and RNA.
Write such reactions and facts about glucose which cannot be explained by its open chain structure.
State reasons for the following:
(i) pKb value for aniline is more than that for methylamine.
(ii) Ethylamine is soluble in water whereas aniline is not soluble in water.
(iii) Primary amines have higher boiling points than tertiary amines.Rearrange the compounds of each of the following sets in order of reactivity towards SN2 displacement:
(i) 2-Bromo-2-methylbutane, 1-Bromopentane, 2-Bromopentane
(ii) 1-Bromo-3-methylbutane, 2-Bromo-2-methylbutane, 3-Bromo-2-methylbutane
(iii) 1-Bromobutane, 1-Bromo-2, 2-dimethylpropane, 1-Bromo-2-methylbutaneHow would you obtain the following?
(i) Benzoquinone from phenol
(ii) 2-methyl propan-2-ol from methyl-magnesium bromide
(iii) Propane-2-ol from propene
Write the names and structures of the monomers of the following polymers:
(i) Buna-S
(ii) Dacron
(iii) NeopreneWhat are the following substances? Give one example of each.
(i) Food preservatives
(ii) Synthetic detergents
(iii) Antacids Long Answer Type
Long Answer Type(a) Draw the structures of the following molecules:
(i) (HPO3)3
(ii) BrF3
(b) Complete the following chemical equations:
(i) HgCl2 + PH3-->
(ii) SO3 + H2SO4 -->
(iii) XeF4 + H2O -->
OR
(a) What happens when?
(i) Chlorine gas is passed through a hot concentrated solution of NaOH?
(ii) Sulphur dioxide gas is passed through an aqueous solution of a Fe (III) salt?
(b) Answer the following:
(i) What is the basicity of H3PO3 and why?
(ii) Why does fluorine not play the role of a central atom in inter-halogen compounds?
(iii) Why do noble gases have very low boiling points?
(a) What type of a battery is lead storage battery? Write the anode and cathode reactions and the overall cell reaction occurring in the operation of a lead storage battery.
(b) Calculate the potential for half-cell containing 0.10 M K2Cr2O7 (aq), 0.20 M Cr3+(aq) and 1.0 x 10-4 M H+ (aq)
The half-cell reaction is 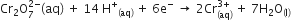
And the standard electrode potential is given as E0 = 1.33 V.
OR
(a) How many moles of mercury will be produced by electrolysing 1.0 M?
Hg (NO3)2 solution with a current of 2.00 A for 3 hours?
[Hg (NO3)2 = 200.6 g mol-1]
(b) A voltaic cell is set up at 25°C with the following half-cells Al3+ (0.001 M) and Ni2+ (0.50 M). Write an equation for the reaction that occurs when the cell generates an electric current and determine the cell potential.

(a) A lead storage battery is a secondary battery.
The following chemical equations take place in a lead storage battery.
: 
When a battery is charged, the reverse of all these reactions takes place.
Hence, on charging, PbSO4(s) present at the anode and cathode is converted into Pb(s) and PbO2(s) respectively.
b) 
Or
(a) Quantity of electricity passed = (2A) x (3 x 60 x 60s) = 21600 C
Thus, 2F i.e. 2 x 96500 C deposit Hg = 1 mole 21600 C will deposit Hg

= 0.11 mole
Or
At anode: Al (s) --> Al3+ (aq) + 3e-] x2
At cathode: Ni2+ + 2e- --> Ni(s) ] x3
Cell reaction: 2Al(s) + 3Ni2+(aq) ---> 2Al3+(aq) + 3Ni (s)
Applying nernst equation to the above cell reaction 
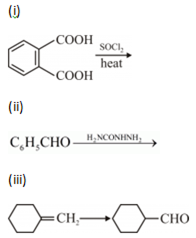
(a) Illustrate the following name reactions:
(i) Cannizzaro’s reaction
(ii) Clemmensen reduction
(b) How would you obtain the following?
(i) But-2-enal from ethanal
(ii) Butanoic acid from butanol
(iii) Benzoic acid from ethylbenzene
OR
(a) Given chemical tests to distinguish between the following:
(i) Benzoic acid and ethyl benzoate
(ii) Benzaldehyde and acetophenone
(b) Complete each synthesis by giving missing reagents or products in the following: