 Short Answer Type
Short Answer TypeAlthough chlorine is an electron withdrawing group, yet it is ortho-, Para-directing in electrophilic aromatic substitution reactions. Explain why it is so?
Draw the structure and name the product formed if the following alcohols are oxidised. Assume that an excess of oxidising agent is used.
(i) CH3CH2CH2CH2OH
(ii) 2-butenol
(iii) 2-methyl-1-proponal
Write chemical equation for the following conversions:
(i) Nitrobenzene to benzoic acid.
(ii) Benzyl chloride to 2-phenylethanamine.
(iii) Aniline to benzyl alcohol.
What are the following substances? Give one example of each one of them.
(i) Tranquilizers
(ii) Food preservatives
(iii) Synthetic detergents
Illustrate the following name reaction giving suitable example in each case:
(i) Clemmensen reduction
(ii) Hell-Volhard-Zelinsky reaction
Give simple tests to distinguish between the following pairs of compounds.
(i) Pentan-2-one and Pentan-3-one
(ii) Benzaldehyde and Acetophenone
(iii) Phenol and Benzoic acid
Illustrate the following reactions giving a suitable example for each.
(i) Cross aldol condensation
(ii) Decarboxylation
Give simple tests to distinguish between the following pairs of compounds
(i) Pentan-2-one and Pentan-3-one
(ii) Benzaldehyde and Acetophenone
(iii) Phenol and Benzoic acid
i) Pentan-2-one and pentan-3-one can be distinguished by iodoform test.
Pentan-2-one is a methyl ketone. Thus, it responds to this test. But pentan-3-one not being a methyl ketone does not respond to this test.
ii) Benzaldehyde (C6H5CHO) and acetophenone (C6H5COCH3) can be distinguished by iodoform test. Acetophenone, being a methyl ketone on treatment with I2/NaOH undergoes iodoform reaction to give a yellow ppt. of iodoform. On the other hand, Benzaldehyde does not give this test.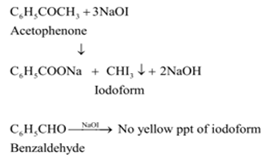
iii) Phenol and benzoic acid can be distinguished by ferric chloride test.
Ferric chloride test:
Phenol reacts with neutral FeCl3 to form ferric phenoxide complex giving violet coloration.
6C6H5OH + FeCl3 ---> [Fe (OC6H5)6]3-+3H++3Cl-
Phenol iron-phenol complex
(Violet color)
But benzoic acid reacts with neutral FeCl3 to give a buff-coloured precipitate of ferric benzoate.![]()
