 Short Answer Type
Short Answer TypeComplete the following chemical equations:
(i) Ca3P2 + H2O -->
(ii) Cu + H2SO4(conc.)-->
Arrange the following in the order of the property indicated by each set:
(i) HF, HCl, HBr, HI - increasing bond dissociation enthalpy.
(ii) H2O, H2S, H2Se, H2Te- increasing acidic character.
Write the IUPAC name of the complex [Cr(NH3)4Cl2]+. What type of isomerism does it exhibit?
In reference to Freundlich adsorption isotherm, write the expression for adsorption of gases on solids in the form of an equation.
Based on the type of particles of dispersed phase, give one example each of associated colloid and multimolecular colloid.
Account for the following:
(i) PCl5 is more covalent than PCl3.
(ii) Iron on reaction with HCl forms FeCl2 and not FeCl3.
(i) Greater the positive oxidation state of the central metal atom, greater is its polarising power and thus more is the covalent character of the bond formed between the central metal atom and other atoms.
In PCl5, the central metal atom, P is in +5 oxidation state, while in PCl3, it is in +3 oxidation state. Therefore, PCl5 is more covalent than PCl5.
(ii) Iron reacts with hydrochloric acid in the following manner, resulting in the release of dihydrogen gas.
Fe(s) + 2HCl(aq) ---> FeCl2 (aq) + H2 (g)
The liberated dihydrogen gas may react with the available oxygen and gets converted to a water molecule. This diminishes the chances of oxidation of ferrous chloride to ferric chloride. As a result, FeCl3 is not formed.
(iii) In ozone, the three oxygen atoms are arranged to form a bent shaped structure. The central oxygen atom makes a single bond with one of the terminal oxygen atoms and a double bond with the other terminal oxygen atom. But the electrons of the double bond are delocalised over all the three oxygen atoms. Due to which the single and the double bond are not entirely pure but are the resonance hybrids of single and double bond respectively, giving rise to the O-O bond distance as the average bond distance of the single and double bond.
The resonance structure of the ozone is given below: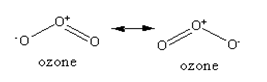
The following data were obtained during the first-order thermal decomposition of SO2Cl2 at a constant volume:
SO2Cl2(g)---> SO2(g) + Cl2(g)
|
Experiment |
Time/s-1 |
Total pressure/atm |
|
1 |
0 |
0.4 |
Calculate the rate constant. (Given : log 4 = 0.6021, log 2 = 0.3010)
