 Short Answer Type
Short Answer TypeGive reasons:
(i)Mn shows the highest oxidation state of +7 with oxygen but with fluorine, it shows the highest oxidation state of +4.
(ii)Transition metals show variable oxidation states.
(iii)Actinoids show irregularities in their electronic configurations.
(i) Name the method of refining of metals such as Germanium.
(ii)In the extraction of Al, impure Al2O3 is dissolved in conc. NaOH to form sodium aluminate and leaving impurities behind. What is the name of this process?
(iii)What is the role of coke in the extraction of iron from its oxides?
(a) For the complex [Fe(CN)6]3–, write the hybridization type, magnetic character and spin nature of the complex. (At. number : Fe = 26).
(b) Draw one of the geometrical isomers of the complex [Pt(en)2Cl2]2+ which is optically active.
Out of 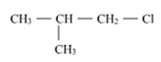 and
and 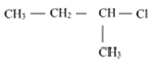 which is more reactive towards
which is more reactive towards
SN 1 reaction and why?
In the SN1 reaction the formation of carbocation is the rate determining step, and also the stability of carbocation would determine its reactivity.
The order of stability of carbocation is given as Tertiary>Secondary> Primary> methyl.
Here, 1-chloro-1-methylpropane would form a secondary carbocation, while 1-chloro-2- methylpropane would form a primary carbocation, which is less stable than secondary carbocation. Hence, reactivity towards the SN1 reaction would be higher for 1-chloro-1- methylpropane.
Given reasons:
(i)C–Cl bond length in chlorobenzene is shorter than C–Cl bond length in CH3–Cl.
(ii)The dipole moment of chlorobenzene is lower than that of cyclohexyl chloride.
(iii)SN1 reactions are accompanied by racemization in optically active alkyl halides.
(i) Write the name of two monosaccharides obtained on hydrolysis of lactose sugar.
(ii) Why Vitamin C cannot be stored in our body?
(iii) What is the difference between a nucleoside and nucleotide?
