 Long Answer Type
Long Answer TypeCompare any five features of Monthly Income Scheme( MIS) and National Saving Certificate (NSC).
Describe five malpractices each of shopkeepers and manufacturers which the consumers often face.
Mahima plays basketball. Elaborate four important features she checks while selecting the fabric for her sports dress? Also, state six instructions she should give to the tailor for satisfactory workmanship for this dress?
List two main ingredients which are used for making a detergent. Write in detail how they help to remove dirt from clothes. Comment on cleaning quality of the soap/ detergent you are using to wash your clothesat home. In your opinion, what is the reason for your satisfaction/ dissatisfaction.
Detergents contain:
The process of removing dirt:
The cleansing action of soap is due to emulsification and micelle formation. Soaps are sodium salts of higher fatty acids such as sodium stearate, C17H35COO–Na+, which ionises as
The anionic head of stearate ion (—COO–) is hydrophobic in nature and has a great affinity for water, while the hydrocarbon part (C17H35) is hydrophobic in nature and great affinity for oil grease etc.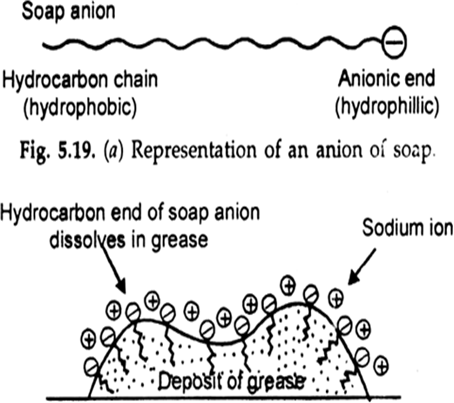
When soap is added to water containing dirt, the soap molecules surround to dirt particles in such a manner that their hydrophobic parts attached to the dirt molecules and the hydrophilic parts point away from the dirt molecule.
This is known as micelle formation thus we can say that the polar group dissolves in water while the non-polar group dissolves in the dirt particles. Now these micelles are negatively charged they do not coalesce and a stable emulsion is formed.
| Soaps | Detergents |
| Cleansing quality of soap is dissatisfactory. | Cleaning quality of detergent is satisfactory. |
| Soap is not effective in cold water | Detergent dissolves in cold and hot water. |
| More water is required for rinsing. | Less water is required for rinsing |
