 Short Answer Type
Short Answer TypeWhy does sun appear red at sunrise and sunset?
Sun appears red at sunrise and sunset because of least scattering of red light as it has the lowest wavelength.
As per Rayleigh scattering, the amount of light scattered 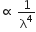 .
.
A ray PQ incident normally on the refracting face BA is refracted in the prism BAC made of material of refractive index 1.5. Complete the path of ray through the prism. From which face will the ray emerge ? Justify your answer.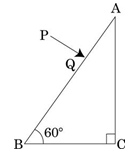
Calculate the de-Broglie wavelength of the electron orbitting in the n=2 state of hydrogen atom.
Define ionisation energy.
How would the ionisation energy change when electron in hydrogen atom is replaced by a particle of mass 200 times that of the electron but having the same charge ?
Calculate the shortest wavelength of the spectral lines emitted in Balmer series. [ Given Rydberg constant, R = 107 m-1]
(i) State law of Malus.
(ii) Draw a graph showing the variation of intensity (I) of polarised light transmitted by an analyser with angle ( ) between polarizer and analyser.
) between polarizer and analyser.
(iii) What is the value of refractive index of a medium of polarising angle 60o?
Sketch the graphs showing variation of stopping potential with frequency of incident radiations for two photosensitive materials A and B having threshold frequencies  A >
A >  B.
B.
(i) In which case is the stopping potential more and why?
(ii) Does the slope of the graph depend on the nature of the material used? Explain.
a) Write the basic nuclear process involved in the emission of beta plus in a symbolic form, by a radioactive nucleus.
b) In the reactions given below: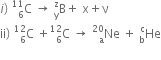
Find the values of x, y and z and a, b and c.
