 Long Answer Type
Long Answer Type
solve the following:
i)What volume of oxygen is required to burn completely 90 dm3 of butane under similar conditions of temperature and pressure?
2C4O10 +13O2 ------> 8CO2 +10H2O
ii)The vapour density of a gas is 8. What would be the volume occupied by 24.0g of the gas at STP.
iii)A vessel contains X number of molecules of hydrogen gas at certain temperature and pressure. How many molecules of nitrogen gas would be present in the same vessel under the same condition of temperature and pressure?
O2 is evolved by heating KClO3 using MnO2 as a catalyst
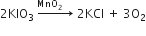
i) Calculate the mass of KClO3 required to produce 6.72 litres of O2 at STP.
[atomic masses of K =39, Cl=35.5, O=16]
ii) Calculate the number of moles of oxygen present in the above volume and also the number of molecules.
iii) Calculate the volume occupied by 0.01 mole of CO2 at STPCopper sulphate solution is electrolyzed using copper electrodes
Study the diagram given below and answer the question that follows: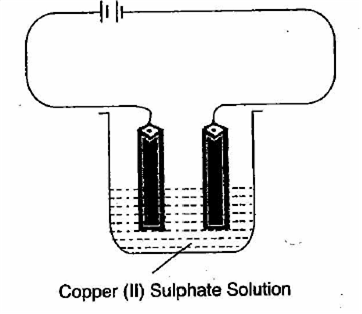
(i) Which electrodes to your left or right is known as the oxidising electrode and why?
(ii) Write the equation representing the reaction that occurs.
(iii) State two appropriate observation for the above electrolysis reaction.
i) The right electrode is known as an oxidising electrode because copper ion gains electron to form copper metal. i.e, reduction takes place.
ii) At cathode: Cu2+ +2e- -----> Cu
At anode: Cu--------> Cu2++2e-
a) Reddish brown metal copper is deposited at the cathode.
b) Blue colour of electrolytic solution I,e. CuSO4 does not fade during the process.
