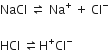Short Answer Type
Short Answer TypeCorrect the following statements:
Ethyl acetate undergoes saponification on treatment with dilute hydrochloric acid.
Correct the following statements:
Aromatic compounds undergo nucleophilic substitution reaction.
Correct the following statements:
Isotopes have the same mass number and atomic number.
The purification of common salt by bubbling hydrogen chloride gas through its solution takes place due to common ion effect.
In both the common salt (NaCl) and hydrogen chloride (HCl), the chloride ion (Cl–) is common so the concentration of Cl– increases on bubbling hydrogen chloride through its solution. This leads the reaction in opposite direction and common salt (NaCl) is precipitated.