 Multiple Choice Questions
Multiple Choice QuestionsWhich of the following sets of quantum numbers is correct for an electron in 4f orbital?
n = 4, I =3, m = +4, s = + 1/ 2
n = 3, I = 2, m = -2, S = + 1/2
n =4, I = 3, m = +1, s = + 1/ 2
n =4, I = 3, m = +1, s = + 1/ 2
Consider the ground state of Cr atom (Z = 24). The number of electrons with the azimuthal quantum numbers I =1 and 2 are respectively
12 and 4
16 and 5
16 and 4
16 and 4
The wavelength of the radiation emitted, when in hydrogen atom electron falls from infinity to stationary state 1, would be (Rydberg constant = 1.097×107 m-1)
91 nm
9.1×10-8 nm
406 nm
406 nm
The correct order of bond angles (smallest first) in H2S, NH3, BF3 and SiH4 is
H2S < SiH4 < NH3 < BF3
H2S < NH3 < BF3 < SiH4
NH3 < H2S < SiH4 < BF3
NH3 < H2S < SiH4 < BF3
Which one the following sets of ions represents the collection of isoelectronic species?
K+ , Ca2+, Sc3+, Cl-
Na+ , Mg2+, Al3+, Cl-
K+ , Cl- , Mg2+, Sc3+
K+ , Cl- , Mg2+, Sc3+
Among Al2O3, SiO2, P2O3 and SO2 the correct order of acid strength is
SO2 < P2O3 < SiO2 < Al2O3
Al2O3 < SiO2 < P2O3 < SO2
Al2O3 < SiO2 < SO2 < P2O3
Al2O3 < SiO2 < SO2 < P2O3
The bond order in NO is 2.5 while that in NO+ is 3. Which of the following statements is true for these two species?
Bond length in NO+ is greater than in NO
Bond length is unpredictable
Bond length in NO+ in equal to that in NO
Bond length in NO+ in equal to that in NO
The formation of the oxide ion O2-(g) requires first an exothermic and then an endothermic step as shown below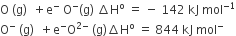
Oxygen is more electronegative
O- ion has comparatively larger size than oxygen atom
O- ion will tend to resist the addition of another electron
O- ion will tend to resist the addition of another electron
The states of hybridization of boron and oxygen atoms in boric acid (H3BO3) are respectively
sp2 and sp2
sp3 and sp3
sp3 and sp2
sp3 and sp2