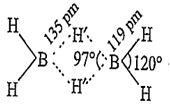Multiple Choice Questions
Multiple Choice QuestionsThe correct order of the acidity of following acids is-
CH3COOH, HCOOH, Cl-CH2COOH, F-CH2COOH, (Cl2)CHCOOH
Cl2CHCOOH > F-CH2COOH > Cl-CH2COOH > HCOOH > CH3COOH
F-CH2COOH > Cl-CH2COOH > Cl2CHCOOH > HCOOH > CH3COOH
HCOOH > CH3COOH > Cl-CH2COOH > Cl2CHCOOH > F-CH2COOH
None of the above
Number of α and β emitted in the reaction, 92U238 → 82Pb206 are
6α and 8β
8α and 6β
6α and 4β
4α and 6β
According to MOT which of the following is correct for potassium hexa cyano ferrate (III)?
It is octahedral complex.
t2g orbital contains the e- of metal.
For iron, overlapping between empty orbitals and ligand orbitals takes place.
All of the above
Which of the following statement is correct?
Basic nature increases on increasing pH
Basic nature decreases on increasing pH
Acidic nature increases on increasing pH
None of the above
Which of the following is an electron deficient compound?
B2H6
NH3
C2H6
CCl4
A.
B2H6
B2H6 is an electron deficient compound in which B is sp3-hybridised state. It has the following H-bridged structure.