 Multiple Choice Questions
Multiple Choice QuestionsThe comparatively high b.pt. of HF is due to
high reactivity of fluorine
small size of hydrogen atom
formation of hydrogen bonds and consequent association
high IE of fluorine
Phenols are more acidic than alcohols because
phenoxide ion is stabilised by resonance
phenols are more soluble in polar solvents
phenoxides ions do not exhibit resonance
alcohols do not lose H atoms at all
Formalin is the commercial name of
formic acid
fluoroform
40% aqueous solution of methanal
para formaldehyde
The aldol condensation of CH3 -CHO results is the formation of
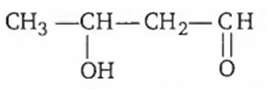
![]()
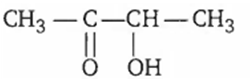
A.
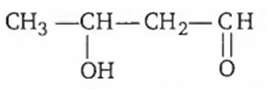
Two molecules of acetaldehyde gives aldol on aldol condensation
