 Multiple Choice Questions
Multiple Choice QuestionsWhen same quantity of electricity is passed through aqueous AgNO3 and H2SO4 solutions connected in series, 5.04 × 10-2 g of H2 is liberated. What is the mass of silver (in grams) deposited ? (Eq. wts. of hydrogen = 1.008, silver = 108)
54
0.54
5.4
10.8
When electric current is passed through acidified waterfor 1930 s, 1120 mL of H2 gas is collected (at STP) at the cathode. What is the current passed in amperes ?
0.05
0.50
5.0
50
Which one of the following reactions does not occur?
F2 + 2Cl- → 2F- + Cl2
Cl2 + 2F- → 2Cl- + F2
Br2 + 2I- → 2Br- + I2
Cl2 + 2Br- → 2Cl- + Br2
B.
Cl2 + 2F- → 2Cl- + F2
With progressive increase in atomic number, the reduction potential of halogens decreases, thus oxidising power also decreases. Hence, a halogen with lower atomic number will oxidise the halide ion of higher atomic number and therefore, will liberate them from their salt solution.
Hence, the reaction
Cl2 + 2F- → 2Cl- + F2
For a reversible reaction, A B, which one of the following statements is wrong from the given energy profile diagram?
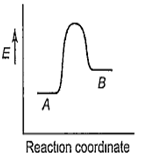
Activation energy of forward reaction is greater than backward reaction
The forward reaction is endothermic.
The threshold energy is less than that of activation energy.
The energy of activation of forward reaction is equal to the sum of heat of reaction and the energy of activation of backward reaction.
Acetone on addition to methyl magnesium bromide forms a complex, which on decomposition with acid gives X and Mg(OH)Br. Which one of the following is X?
CH3OH
(CH3)3OH
(CH3)2CHOH
CH3CH2OH
Identify A and B in the following reaction
A - HI + red P; B - LiAlH4
A - Ni/ ; B - LiAlH4
A - LiAlH4 ; B - HI + red P
A - Pd- BaSO4 ; B - Zn - HCl
Match the following:
| List - I | List - II |
| A. Oxyhaemoglobin | i. Analgesic |
| B. Aspirin | ii. Oxygen carrier |
| C. Haemoglobin | iii. Photosynthesis |
| D. Chlorophyll |
iv. Oil of winter green v. Fe2+ paramagentic |
The correct match is
A - v; B - i; C - ii; D - iii
A - iv; B - ii; C - i; D - iii
A - iii; B - i; C - ii; D - iv
A - v; B - ii; C - iii; D - i
If is the weight average molecular weight and is the number average molecular weight of a polymer, the poly disparity index (PDI) of the polymer is given by
Hydrolysis of sucrose with dilute aqueous sulphuric acid yields-
1 : 1 D-(+)-glucose; D-(-)-fructose
1 : 2 D-(+)-glucose; D-(-)-fructose
1 : 1 D-(-)-glucose; D-(+)-fructose
1 : 2 D-(-)-glucose; D-(+)-fructose
