 Multiple Choice Questions
Multiple Choice QuestionsWhich one of the following sets correctly represents the increase in the paramagnetic property of the ions?
Cu2+ > V 2+ > Cr2+ > Mn2+
Cu2+ < Cr2+ < V 2+ < Mn2+
Cu2+ < V 2+ < Cr2+ < Mn2+
V 2+ < Cu2+ < Cr2+ < Mn2+
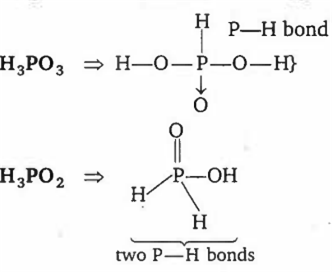
Which pair of oxyacids of phosphorus contains 'P - H' bonds?
H3PO4, H3PO3
H3PO5, H4P2O7
H3PO3, H3PO2
H3PO2, HPO3
C.
H3PO3, H3PO2
Aluminium reacts with NaOH and forms compound 'X'. If the coordination number of aluminium in 'X' is 6, the correct formula of X is
[Al(H2O)4(OH)2]+
[Al(H2O)3(OH)3]
[Al(H2O)2(OH)4]-
[Al(H2O)6](OH)3
During the depression in freezing point experiment, an equilibrium is established between the molecules of
liquid solvent and solid solvent
liquid solute and solid solvent
liquid solute and solid solute
liquid solvent and solid solute
Which one of the following is most effective in causing the coagulation of an As2S3 sol?
KCl
AlCl3
MgSO4
K3Fe(CN)6
At 25C, the molar conductances at infinite dilution for the strong electrolytes NaOH, NaCl and BaCl2 are 248 10-4, 126 x 10-4 and 280 x 10-4 Sm2 mol-1 respectively, Ba(OH)2 in Sm2 mol-1 is
52.4 10-4
524
402
262
For a first order reaction at 27C, the ratio of time required for 75% completion to 25% completion of reaction is
3.0
2.303
4.8
0.477
