Multiple Choice Questions
Multiple Choice QuestionsIdentify the statement which is not correct regarding CuSO4
It react with KI to give iodine
It react with KCl to give Cu2Cl2
It react with NaOH and glucose to give Cu2O.
It gives CuO on strongly heating in air.
Transition metals usually exhibit highest oxidation states in their
chlorides
fluorides
bromides
iodides
Which one of the following cells can convert chemical energy of H2 and O2 directly into electrical energy?
Mercury cell
Daniell cell
Fuel cel
Lead storage cell
The activity of an old piece of wood is just 25% of the fresh piece of wood. If t1/2 of C-14 is 6000 yr, the age of piece of wood is
6000 yr
3000 yr
9000 yr
12000 yr
Which of the following gives red colour in Victor Meyer's test?
n-propyl alcohol
Isopropyl alcohol
tert-butyl alcohol
sec-butyl alcohol
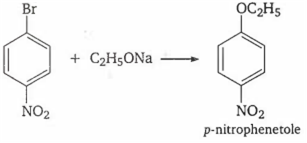
When p-nitrobromobenzene reacts with sodium ethoxide, the product obtained is
p-nitroanisole
ethyl phenyl ether
p-nitrophenetole
no reaction occurs
C.
p-nitrophenetole
In general aryl halides are highly stable and do not take part in Williamson's synthesis, but presence of strong electron withdrawing group like NO2 makes the C-X bond weaker and facilitate the substitution of -Br by -OR.