 Multiple Choice Questions
Multiple Choice QuestionspH value of which one of the following is not equal to one?
0.1 M HNO3
0.05 M H2SO4
0.1 M CH3COOH
50 cm3 of 0.4 M HCl + 50 cm3of 0.2 M NaOH
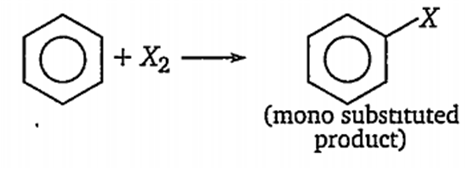
Which one of these is not true for benzene?
It forms only one type of monosubstituted product
There are three carbon-carbon single bonds and three carbon-carbon double bonds
The heat of hydrogenation of benzene is less than the theoretical value
The bond angle between the carbon-carbon bonds is 120°
B.
There are three carbon-carbon single bonds and three carbon-carbon double bonds
The structure of benzene is
![]()
Since, the bond order is in between single and double bond, thus it contains delocalised π bonds. Hence, it is not possible to obtain number ofsingle and double bonds in benzene.
Generally, the first ionization energy increases along a period. But there are some exceptions. One which is not an exception is
N and O
Na and Mg
Mg and Al
Be and B
Which one of the following conformations of cyclohexane is the least stable?
Half-chair
Boat
Twisted-boat
Chair
Chloroacetic acid is a stronger acid than acetic acid. This can be explained using
-M effect
- I effect
+M effect
+I effect
For alkali metals, which one of the following trends is incorrect?
Hydration energy : Li > Na > K > Rb
Ionization energy : Li > Na > K > Rb
Density : Li < Na < K < Rb
Atomic size : Li < Na < K < Rb
A dibromo derivative of an alkane reacts with sodium metal to form an alicyclic hydrocarbon. The derivative is
1, 1-dibromopropane
2, 2-dibromobutane
1, 2-dibromoethane
1, 4-dibromobutane
In the electrolytic refining of zinc
graphite is at the anode
the impure metal is at the cathode
the metal ion get reduced at the anode
acidified zinc sulphate is the electrolyte
In chromite ore, the oxidation number of iron, and chromium are respectively
+3,+2
+3,+6
+2,+6
+2,+3
