 Multiple Choice Questions
Multiple Choice QuestionsThe following data is obtained during the first order thermal decomposition of 2A(g)→ B(g) +C(s) at constant volume and temperature.
| S.No | Time | Total pressure in Pascal |
| 1. | At the end of 10 min | 300 |
| 2. | After completion | 200 |
The rate constant in min-1
0.0693
6.93
0.00693
69.3
Which one of the following is an example for homogeneous catalysis?
Manufacture of sulphuric acid by Contact process
Manufacture of ammonia by Haber's process
Hydrolysis of sucrose in presence of dilute hydrochloric acid
Hydrogenation of oil
A white crystalline salt A reacts with dilute HCl to liberate a suffocating gas B and also forms a yellow precipitate. The gas B turns potassium dichromate acidified with dilute H2SO4 to a green coloured solution C. A, B, C are respectively
Na2SO3, SO2, Cr2(SO4)3
Na2S2O3, SO2, Cr2(SO4)3
Na2SO2 , SO2, Cr2(SO4)3
Na2SO4, SO2, Cr2(SO4)3
Molecules of a noble gas do not possess vibrational energy because a noble gas
is monoatomic
is chemically inert
has completely filled shells
is diamagnetic
E1, E2, E3 are the emf values of the three galvanic cells respectively.
Which one of the following is true?
E1> E2 >E3
E2 >E3 >E1
E3 >E2 > E1
E1> E3 >E2
Out of the compounds below the vapour pressure of (B) at a particular temperature is
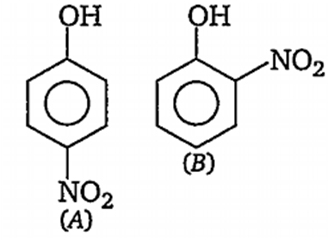
higher than that of (A)
lower than that of (A)
higher or lower than (A), depending on the size of the vessel
same as that of (A)
An oxygen containing organic compound upon oxidation forms a carboxylic acid as the only organic product with its molecular mass higher by 14 units. The organic compound is
an aldehyde
a primary alcohol
a secondary alcohol
a ketone
The compound obtained when acetaldehyde reacts with dilute aqueous sodium hydroxide exhibits
geometrical isomerism
optical isomerism
neither optical nor geometrical isomerism
both optical and geometrical isomerism
B.
optical isomerism
When acetaldehyde is treated with aqueous sodium hydroxide solution, it undergoes aldol condensation (because of the presence of α-H atom) as.
Since, the aldol formed contains one asymmetric carbon atom, it will show optical
isomerism. On heating, aldol loses water and gives an alkene.
The correct sequence of reactions to convert p-nitrophenol into quinol involves
reduction, diazotization and hydrolysis
hydrolysis, diazotization and reduction
hydrolysis, reduction and diazotization
diazotization, reduction and hydrolysis
