 Multiple Choice Questions
Multiple Choice QuestionsThe letter 'D' in D-glucose signifies
configuration at all chiral carbons
dextrorotatory
that it is a monosaccharide
configuration at a particular chiral carbon
Reaction of methyl bromide with aqueous sodium hydroxide involves
racemisation
SN 1 mechanism
retention of configuration
SN 2 mechanism
Benzaldehyde and acetone can be best distinguished using
Fehling's solution
Sodium hydroxide solution
2, 4-DNP
Tollen's reagent
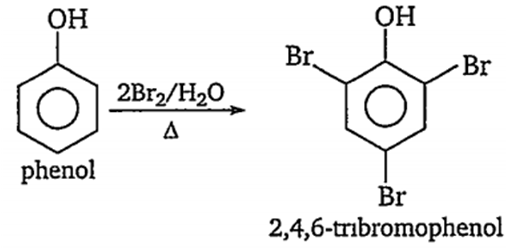
Phenol forms a tribromo derivative, 'X' is
bromine in benzene
bromine in water
potassium bromide solution
bromine in carbon tetrachloride at 0°C
One mole of an organic compound A with the formula C3H8O reacts completely With two moles of HI to form X and Y. When Y is boiled with aqueous alkali it forms Z. Z answers the iodoform test. The compound A is
propan-2-ol
propan-1-ol
ethoxyethane
methoxyethane
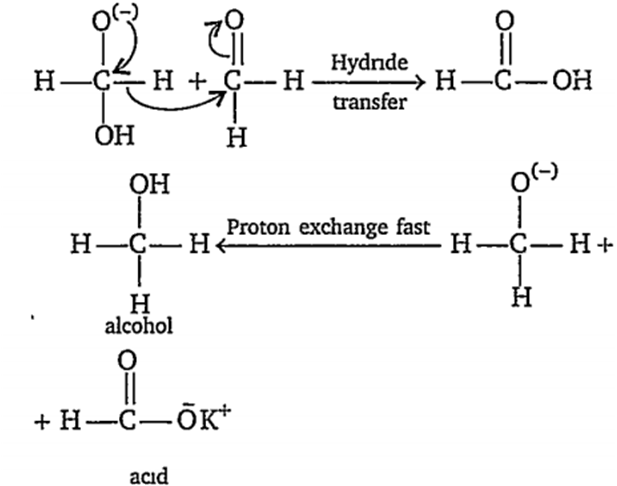
The correct sequence ofsteps involved in the mechanism of Cannizaro's reaction is
nucleophilic attack, transfer of H- and transfer of H+
transfer of H-, transfer of H+ and nucleophilic attack
transfer if H+, nucleophilic attack and transfer of H-
electrophilic attack by OH-, transfer of H+ and transfer of H-
A.
nucleophilic attack, transfer of H- and transfer of H+
The Cannizaro reaction is as
The mechanism of Cannizaro reaction is as
Step Ist : Attack of nucleophile OH- to the carbonyl carbon
Step Ind : The transfer of hydride ion from anion (I) to second molecule of aldehyde and finally rapid transfer of proton takes place.
The IUPAC name of ![]()
2-methyl-3-bromohexanal
3-bromo-2-methylbutanal
2-methyl-3-bromobutanal
3-bromo-2-methylpentanal
Which one of the following forms propane nitrile as the major product?
Ethyl bromide + alcoholic KCN
Propyl bromide + alcoholic KCN
Propyl bromide + alcoholic AgCN
Ethyl bromide + alcoholic AgCN
