 Multiple Choice Questions
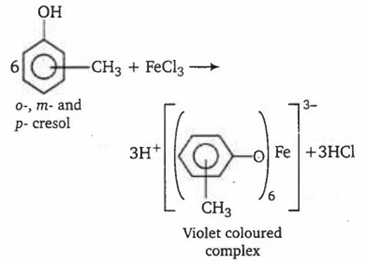
Multiple Choice QuestionsTwo aromatic compounds having formula C7H8O which are easily identifiable by FeCl3 solution test (violet colouration) are
o-cresol and benzyl alcohol
m-cresol and p-cresol
o-cresol and p-cresol
methyl phenyl ether and benzyl alcohol
A.
o-cresol and benzyl alcohol
o-, m- and p- cresol all contain phenolic group, thus they give violet colouration with FeCl3 whereas benzylalcohol and methyl phenyl ether donot contain phenolic group, hence give no colouration with FeCl3. Hence the pair of compounds which are identifiable by FeCl3 is o-cresol and benzyl alcohol.
The ease of dehydrohalogenation of alkyl halide with alcoholic KOH is
3° < 2° < 1°
3° > 2° > 1°
3° < 2° > 1°
3° > 2° < 1°
The correct order of decreasing acidity of nitrophenols will be
m-nitrophenol > p-nitrophenol > o-nitrophenol
o-nitrophenol > m-nitrophenol > p-nitrophenol
p-nitrophenol > m-nitrophenol > o-nitrophenol
p-nitrophenol > o-nitrophenol > m-nitrophenol
An electric current is passed through an aqueous solution of a mixture of alanine (isoelectric point 6.0), glutamic acid (3.2) and arginine (10. 7) buffered at pH 6. What is the fate of the three acids?
Glutamic acid migrates to anode at pH 6. Arginine is present as a cation and migrates to the cathode. Alanine in a dipolar ion remains uniformly distributed in solution
Glutamic acid migrates to cathode and others remain uniformly distributed in solution.
All three remain uniformly distributed in solution.
All three move to cathode
In aqueous solution glucose remains as
only in open chain form
only in pyranose form
only in furanose form
n all three forms in equilbrium
Reaction of formaldehyde and ammonia gives
hexamethylene tetramine
bakelite
urea
triethylene tetramine
Paracetamol is
methyl salicylate
phenyl salicylate
N-acetyl p-amino phenol
acetyl salicylic acid
Which of the following compounds is not formed in iodoform reaction of acetone?
CH3COCH2I
ICH2COCH2I
CH3COCHI2
CH3COCI3
