 Multiple Choice Questions
Multiple Choice QuestionsWhich one of the pairs C6H5COOMe and 2,6-(Me)2C6H3COOMe, C6H5COOMe and C6H5CH2COOMe, CH3COCH3, and CF3COCH3, p-NO2C6H4COOMe and p- MeOC6H4COOMe in that order, will react faster than the other with a nucleophile at the carbonyl carbon?
C6H5COOMe,C6H5CH2COOMe, CF3COCH3, p-NO2C6H4COOMe
2,6-(Me)2C6H3COOMe, C6H5CH2COOMe, CH3COCH3, p-NO2C6H4COOMe
2,6-(Me)2C6H3COOMe, C6H5CH2COOMe, CH3COCH3 , p- MeOC6H4COOMe
C6H5COOMe,C6H5CH2COOMe, CF3COCH3, p- MeOC6H4COOMe
Which of the following chemicals does/do not generate shiny deposits on add mixture with ammoniacal silver nitrate?
Acetaldoxime
Cyclohexanone
Butyraldehyde
both (a) or (b)
Wacker process is an industrial process for the oxidation of terminal alkenes. Which of the following represents a true statement about the product?
The product is an aldehyde with same number of carbon atoms as in the alkene
The product is a ketone with same number of carbon atoms as in the alkene
The product is an oxirane with same number of carbon atoms as in the alkene
Both (a) and (b)
Which of the following is/are formed on reaction of benzaldehyde with aqueous NaOH followed by acidification with dilute hydrochloric acid?
Benzoic acid
Benzyl alcohol
Benzoic acid and benzyl alcohol
p-hydroxybenzaldehyde
Which of the following is the correct IUPAC name of CH3CHBrCCCHO?
4-bromo-2-pentynal
2-bromo-3-pentynal
1-bromo-1-methyl-2-butynal
4-bromo-4-methyl-butynal
Which one is the correct IUPAC name of the following compound?

1-chloro-5-hydroxycydohexane
2-chloro-4-hydroxycyclohexane
3-chloro-3-cyclohexenol
5-hydroxycydohexenyl chloride
Which of the following represents the correct decreasing order of acidity of the following compounds?
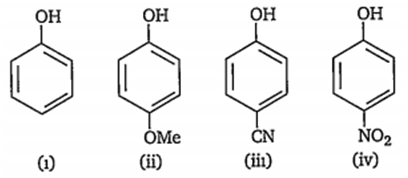
ii > i > iii > iv
iv > iii > ii > i
iii > iv > i > ii
iv > iii > i > ii
D.
iv > iii > i > ii
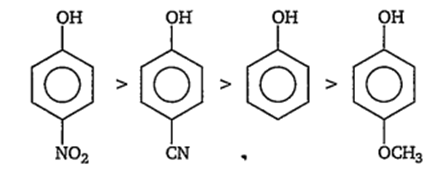
Electron withdrawing groups (-NO2,-CN) stabilize the phenoxide ion by dispersing the negative charge relative to phenol and increase the acidity of phenol, whereas electron donating groups (-OCH3) destabilize the phenoxide ion by intensifying the negative charge relative to phenol and tend to decrease the acid strength. Thus, the order of acidity is
The configurations at C1 and C2 in the following compound are, respectively
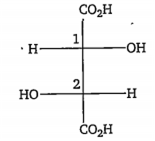
R and R
R and S
S and R
S and S
The following species generates a highly stable radical on exposure to 100 W bulb light. Which one of the following represents this stable radical?
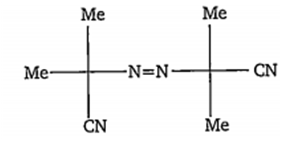
Me2(CN)
H3
Me2C(CN)N=
Which of the following compounds will give red colour on Lassaigne test?
NaCNS
NH2CSNH2 (thiourea)
p-NH2C6H4SO3H (p- aminobenzene sulphonic acid)
all of the above
