 Multiple Choice Questions
Multiple Choice QuestionsThe condition of spontaneity of a process is
lowering of entropy at constant temperature and pressure
lowering of Gibbs free energy of system at constant temperature and pressure
increase of entropy of system at constant temperature and pressure
increase of Gibbs free energy of the universe at constant temperature and pressure
The increasing order of O - N - O bond angle in the species NO2, NO2+ and NO2- is
NO2- < NO2 < NO2+
NO2 < NO2- < NO2+
NO2+ < NO2- < NO2
NO2 < NO2+ < NO2-
A.
NO2- < NO2 < NO2+
Among all the given options, option a is correct. igher the number of lone pair of electrons (greater is repulsion) lower is the bond angle and vice-versa. In NO2- one lone pair of electron is present while in NO2+ no lone pair of electron is present. Hence, the correct order of bond angles is NO2- < NO2 < NO2+.
For BCl3, AlCl3 and GaCl3 the increasing order of ionic character is
BCl3 < AlCl3 < GaCl3
GaCl3 < AlCl3 < BCl3
BCl3 < GaCl3 < AlCl3
AlCl3 < BCl3 < GaCl3
1 x 10-3 mole of HCl is added to a buffer solution made up of' 0.01 M acetic acid and 0.01 M sodium acetate. The final pH of the buffer (given, pKa of acetic acid is 4.75 at 25°C)
4.60
4.66
4.75
4.8
The IUPAC name of the compound X is
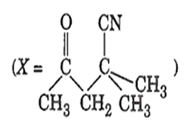
4-cyano-4-methyl-2-oxopentane
2-cyano-2-methyl- 4-oxopentane
2, 2-dimethyl-4-oxopentanenitrile
4-cyano-4-methyl-2-pentanone
(+)-2-chloro-2-phenylethane in toluene racemises slowly in the presence of small amount of SbCl2, due to the formation of
carbanion
carbene
free-radical
carbocation
Nitric acid can be obtained from ammonia via the formations of the intermediate compounds
nitric oxide and nitrogen dioxide
nitrogen and nitric oxide
nitric oxide and dinitrogen pentoxide
nitrogen and nitrous oxide
Correct pair of compounds which gives blue colouration/precipitate and white precipitate, respectively, when their Lassaigne's test is separately done is
NH2NH2, HCl and ClCH2COOH
NH2CSNH2 and PhCH2Cl
NH2NH2, COOH and NH2CONH2

Chlorine gas reacts with red hot calcium oxide to give
bleaching powder and dichlorine monoxide
bleaching powder and water
calcium chloride and chlorine dioxide
calcium chloride and oxygen
2-methylpropane on monochlorination under photochemical condition give
2-chloro-2-methylpropane as major product
(1 : 1) mixture of 1-chloro-2-methylpropane and 2-chloro-2-methyl propane
1-chloro-2-methyl propane as a major product
(1 : 9) mixture of 1-chloro-2-methylpropane and 2-chloro-2-methylpropane
