 Multiple Choice Questions
Multiple Choice QuestionsA positive carbylamine test is given by
I. N, N-dimethylaniline
II. 2, 4-dimethylamine
III. N-methyl-o-methylaniline
IV. p-methylbenzyl amine
(II) and (IV)
(I) and (IV)
(II) and (III)
(I) and (II)
Identify the final product B of the reaction
C6H5COOH + NaHO3 → gas (A) (B)
Here, = C14
H3COOH
C6H5OOH
CH3OOH
HOOCH3
CH3CHO + HCHO
The structure of the compound B is
CH2=CH-CH(OH)-COOH
CH2=CH-CH(CN)-OH
CH3-CH2-CN(CN)OH
CH3--COOH
Phenol + CCl4 + KOH → X;
Which of the following statement is true for X?
It gives effervescence with NaHCO3
Gives silver mirror with Tollen's reagent
Does not give the red colour with FeCl3
All of the above
Out of
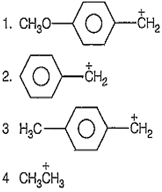
Relative stabilities order is
4 < 2 < 3 < 1
2 > 4 < 3 < 1
4 < 2 < 1 < 3
2 < 4 < 1 < 3
A.
4 < 2 < 3 < 1
Due to the presence of phenyl group the stabihties of the carbocations 1, 2 and 3 are greater than cation 4, (due to resonance). Further the presence of electron-donating groups on phenyl ring increases the stability of carbocation.
Hence, cation 1 and 3 are more stable than cation 2. Moreover - OMe group shows +M effect which is more prominent than +I effect of -Me group. Thus, cation 1 is more stable than cation 3. So, the correct order is, 4 < 2 < 3 < 1.
