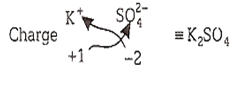Multiple Choice Questions
Multiple Choice QuestionsThe correct increasing order of the acid strength of benzoic acid (I), 4-nitrobenzoic acid (II), 3,4-dinitrobenzoic acid (III) and 4-methoxybenzoic acid (IV) is
I < II < III < IV
II < I < IV < III
IV < I < II < III
IV < II < I < III
Choose the correct order of decreasing basic strength of the following compounds in aqueous solution
(i) C6H5NH2
(ii) C2H5NH2
(iii) NH3
(iv) (CH3)2NH
(i) > (ii) > (iii) > (iv)
(iv) > (ii) > (iii) > (i)
(ii) > (i) > (iii) > (iv)
(iv) > (iii) > (ii) > (i)
The shortest wavelength of the line in hydrogen atomic spectrum of Lyman series when RH = 109678 cm-1 is
1002.7 Å
1215.67 Å
1127.30 Å
911.7 Å
Arrange the following species in the correct order of their stability
C2, Li2, O, He
Li2 < He < O < C2
C2 < O < Li2 < He
He < Li2 < C2 < O
O < C2 < Li2 < He
Molecular formulae and shapes of some molecules are given below. Choose the incorrect match.
NH3 - Trigonal pyramidal
SF4 - Tetrahedral
ClF3 - T-shaped
PCl5 - Triginal bipyramidal
If two moles of an ideal gas at 500 K occupies a volume of 41 L, the pressure of the gas is (R = 0.082 L atm K-1 mol-1)
2 atm
3 atm
4 atm
5 atm
At 273 K, the density of a certain gaseous oxide at 2 atm is same as that of dioxygen at 5 atm. The molecular mass of the oxide (in g mol-1) is
80
64
32
160
Which of the following are isoelectronic species?
(i) NH3
(ii) CH
(iii) NH
(iv) NH
Choose the correct answer from the codes given below.
(i), (ii), (iii)
(ii), (iii), (iv)
(i), (ii), (iv)
(i), (iii), (iv)
The salt of an alkali metal gives violet colour in the flame test. Its aqueous solution gives a white precipitate with barium chloride in hydrochloric acid medium. The salt is
K2SO4
KCl
Na2SO4
K2CO3
A.
K2SO4
The salt gives violet colour in the flame test, which shows the presence of potassium ion (K) in it. Further, the aqueous solution of salt gives white precipitate with BaCl2 in acidic medium, so it must contain sulphate ion (SO) and the precipitate is of BaSO4.
Thus, the salt is