 Multiple Choice Questions
Multiple Choice QuestionsIn a reaction, A + B C + D, D, 40% of B has reacted at equilibrium, when 1 mole of A was heated with 1 mole of B in a 10 L closed vessel. The value of Kc is
0.44
0.18
0.22
0.36
If the ionc product of Ni(OH)2 is 1.9× 10-15, the molar solubility of Ni(OH)2 in 1.0 M NaOH
1.9 × 10-18 M
1.9 × 10-13 M
1.9 × 10-15 M
1.9 × 10-15 M
C.
1.9 × 10-15 M
Which one of the following elements on doping with germanium, make it a p-type semiconductor?
Boron
Selenium
Arsenic
Germanium
Temporary hardness of water is removed in Clark's process by adding
caustic soda
calgon
borax
lime
Which one of the following correctly represents the variation of electronegatrvity (EN) with atomic number (Z) of group 13 elements?
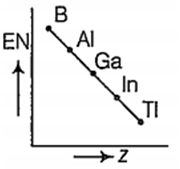
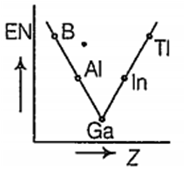
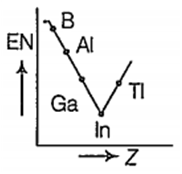
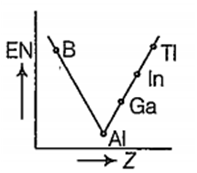
Which one of the following is more readily hydrolysed by SN1 mechanism?
(C6H5)2C(CH3)Br
C6H5CH2Br
C6H5CH(CH3)Br
(C6H5)2CHBr
