 Multiple Choice Questions
Multiple Choice QuestionsWhich one of the following ions has same number of unpaired electrons as those present in V+ ion?
Fe3+
Ni2+
Mn2+
Cr3+
Match the following
| Column I | Column II |
| (A) sp3 | (i) [Co(NH3)6]3+ |
| (B) dsp2 | (ii) [Ni(CO)4] |
| (C) sp3d2 | (iii) [Pt(NH3)2Cl2] |
| (D) d2sp3 | (iv) [CoF6]3- |
| (v) [Fe(CO)5] |
A B C D
(v) (ii) (i) (iii)
A B C D
(ii) (iii) (iv) (v)
A B C D
(ii) (iii) (i) (v)
A B C D
(iii) (ii) (iv) (i)
. Identify Z from the following.
Ethyl acetate
Acetic acid
Propanoic acid
Methyl acetate.
B.
Acetic acid
When Gngnard's reagent is treated with CO2, a magnesium complex is formed, which on further hydrolysis yields monocarboxylic acid.
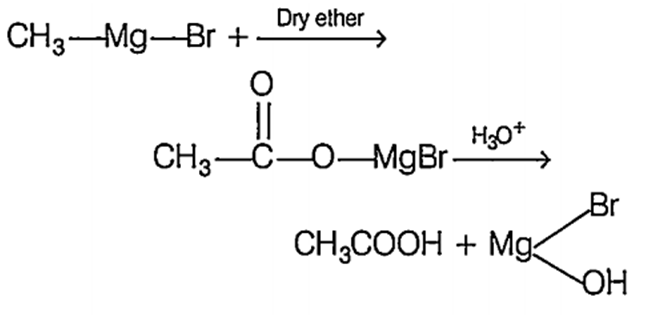
Identify Y and Z inthe above reaction.
| X | Y |
| C6H5OH | H3CCH3 |
| X | Y |
| C2H5I | C6H5CHO |
| X | Y |
| C6H5I | H3CCH2OH |
| X | Y |
| C6H5OH | H3CCH2I |
What are the substances which mimic the natural chemical messengers?
Antibiotics
Antagonists
Agonists
Receptors
Lactose is disaccharide of
α- D - glucose and α- D - fructose
β- D- glucose and β- D-galatose
α- D - glucose and β- D-ribose
α- D - glucose and β- D-galactose
