 Multiple Choice Questions
Multiple Choice QuestionsThe two electrons have the following set of quantum numbers, P =3,2, -2 +1/2, Q =3,0,0 + 1/2. Which of the following statement is true?
P and Q have same energy
P has greater energy than Q
P has lesser energy than Q
P and Q represent same electron
0.06% (w/V) aqueous solution of urea is isotonic with
0.06% glucose solution
0.6% glucose solution
0.01M glucose solution
0.1M glucose solution
Which one of the following conversion results in the change of hybridisation and geometry?
CH4 to C2H2
NH3 to N+H4
BF3 to BF
H2O to H3O+
0.30 g of an organic compound containing C, H and oxygen on combustion yields 0.44 g of CO2 and 0.18 g of H2O. If one mole of compound weighs 60, then molecular formula of the compound is
CH2O
C3H8O
C4H6O
C2H4O2
Plot of Maxwell's distribution of velocities is given below.
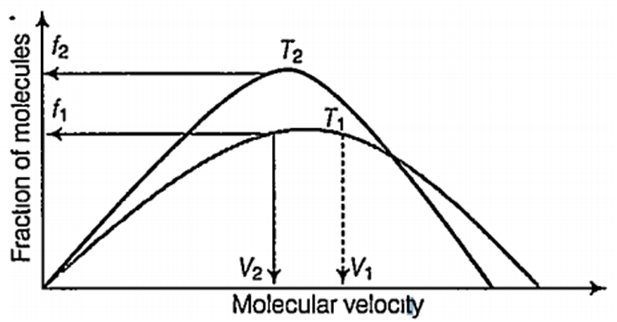
Which of the following is correct about this plot?
T1 < T2
f1 > f2
T1> T2
V1 < V2
The pair of compound which cannot exist together in solution is
NaHCO3 and NaOH
NaHCO3 and H2O
NaHCO3 and Na2CO3
Na2CO3 and NaOH
Using MOT, compare species and choose the inncorrect option.
have higher bond order than
is less stable
is diamagnetic while is paramagnetic
Both are paramagnetic