 Multiple Choice Questions
Multiple Choice QuestionsThe reaction of zinc with dilute and concentrated nitric acid, respectively, produces:
NO2 and NO
NO and N2O
NO2 and N2O
NO2 and N2O
2-chloro-2-methylpentane on reaction with sodium methoxide in methanol yields: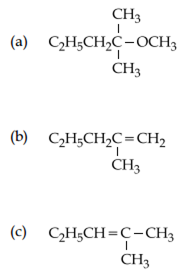
Both a and c
Only c
Both a and b
Both a and b
Which of the following statements about low-density polythene is false?
It is a poor conductor of electricity
Its synthesis requires dioxygen or a peroxide initiator as a catalyst.
It is used in the manufacture of buckets, dustbins etc.
It is used in the manufacture of buckets, dustbins etc.
Which of the following is an anionic detergent?
Sodium lauryl sulphate
Cetyltrimethyl ammonium bromide
Glyceryl oleate
Glyceryl oleate
In the Hofmann bromamide degradation reaction, the number of moles of NaOH and Br2 used per mole of amine produced are:
Four moles of NaOH and two moles of Br2
Two moles of NaOH and two moles of Br2
Four moles of NaOH and one mole of Br2
Four moles of NaOH and one mole of Br2
C.
Four moles of NaOH and one mole of Br2
Hofmann-bromamide degradation reaction is given as
RCONH2 + 4 NaOH + Br2 → RNH2 + Na2CO3 + 2NaBr + 2H2O
Hence four moles of NaOH and one mole of Br2 are used.
The reaction of propene with HOCl(Cl2+H2O) proceeds through the intermediate:
CH3−CH+−CH2−Cl
CH3−CH(OH)−C+H2
CH3−CHCl−C+H2
CH3−CHCl−C+H2
