 Multiple Choice Questions
Multiple Choice QuestionsPoint out the false statement.
Colloidal sols are homogeneous
Colloids carry + ve or - ve charges
Colloids show Tyndall effect
The size range of colloidal particles is 10-1000
Which of the following statements regarding Lanthanides is false?
All lanthanides are solid at room temperature
Their usual oxidation state is +3
They can be separated from one another by ion-exchange method
Ionic radii of trivalent lanthanides steadily increases with increase in atomic number
In the solid state, PCl5 exists as
[PCl4]- and [PCl6]+ ions
covalent PCl5 molecules only
[PCl4]+ and [PCl6]-
covalent P2Cl10 molecules only
The acid in which O-O bonding is present is
H2S2O3
H2S2O6
H2S2O8
H2S4O6
C.
H2S2O8
H2S2O8 shows -O-O-(peroxy) bonding following
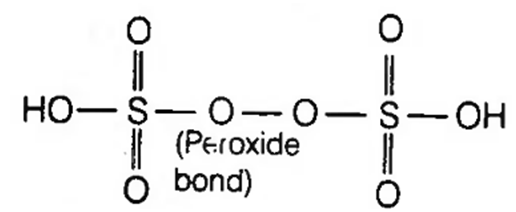
Peroxodisulphuric acid or Marshall's acid
If radium and chlorine combine to form radium chloride, the compound would be
half as radioactive as radium
twice as radioactive
as radioactive as radium
not radioactive
Which of the following arrangements is correct in respect of solubility in water?
CaSO4 > BaSO4 > BeSO4 > MgSO4 > SrSO4
BeSO4 > MgSO4, > CaSO4, > SrSO4, > BaSO4
BaSO4> SrSO4 > CaSO4 >MgSO4 > BeSO4
BeSO4 > CaSO4, > MgSO4 >SrSO4 > BaSO4
If and p are the vapour pressure of the pure solvent and solution and n1 and n2 are the moles of solute and solvent respectively in the solution then the correct relation between p and p° is
Ionic solids with Schottky defect may contain in their structure
cation vacancies only
cation vacancies and interstitial cations
equal number of cation and anion vacancies
anion vacancies and interstitial anions
