 Multiple Choice Questions
Multiple Choice QuestionsZinc oxide loses oxygen on heating according to the reaction
It becomes yellow on heating because
Zn2+ ions and electrons move too interstitial sites and F-centres are created.
Oxygen and electron moves at the crystal and ions become yellow
Zn2+ again combine with oxygen to give yellow oxide
Zn2+ are replaced by oxygen.
When 96.5 C of electricity is passed through a solution of silver nitrate (at wt. of Ag = 107. 8 7 which is approx. 108), the amount of silver deposted is
5.8 mg
10.8 mg
15.8 mg
20.8 mg
The standard reduction potentials at 298 K for the following half reactions are given against each
Which is the strongest reducing agent ?
Zn(s)
Cr(s)
H2(s)
Fe2+ (aq)
following mechanism has been provided.
Thus, rate expression of the above reaction can be written as
r =k [NO2]2[F2]
r= k [NO2] [F2]
r= k [NO2]
r= k [F2]
For a reaction, an aqueous medium, the rate of reaction is given by:
The overall order of reaction is
-1
0
1
2
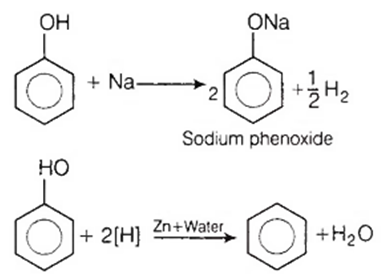
Phenol is bifunctional compound because
It is acidic and contain -OH
It reacts with Na to give phenoxide
It reacts with both Na and Zn to give phenoxide and benzene respectively
Both (a) and (c)
D.
Both (a) and (c)
Which of the following has lowest boiling point?
p -nitrophenol
m -nitrophenol
o-nitrophenol
Phenol
An organic compound X is oxidised by using acidified K2Cr2O7. The product obtained reacts with phenyl hydrazine, but does not answer silver mirror test. The possible structure of X is
CH3COCH3
(CH3)2CHOH
CH3CHO
CH3CH2OH
