 Multiple Choice Questions
Multiple Choice QuestionsPredict the product (B) in the following sequence of reactions
Ethyl benzene A B
Benzaldehyde
Benzoic acid
Benzophenone
Acetophenone
Which one of the following can be prepared by Gabriel phthalimide synthesis?
Aniline
o- toluidine
Benzylamine
N-methylethanamine
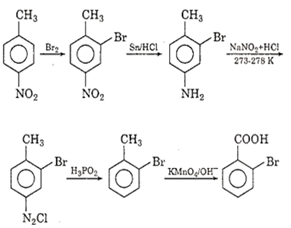
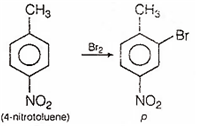
4-nitrotoluene is treated with bromine to get compound 'P' which is reduced with Sn and HCl to get compound 'Q'. 'Q' is diazotised and the product is treated with phosphinic acid to get compound 'R'. 'R' is oxidised with alkaline solution KMnO4 to get the final product. Identify the final product.
2-bromobenzoic acid
2-bromo-4-hydroxybenzoic acid
benzoic acid
3-bromobenzoic acid
A.
2-bromobenzoic acid
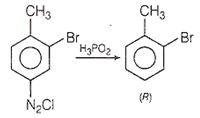
Step 1: Since, NO2 is m-directing group while -CH3 group is o- and p- directing group. Thus, Br group attach at o- position with respect to CH3 and m-position with respect to NO2 group.
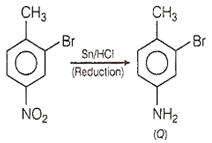
Step 2: On reduction with Sn/ HCl, NO2 group changes to -NH2 group.
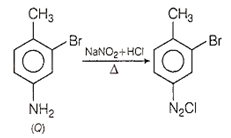
Step 3: On treatment with NaNO2 + HCl; NH2 changes to diazo group, i.e. Q shows diazotisation.
Step 4: On treatment with H3PO2, only diazo group (N2Cl) reacts and gets removed.
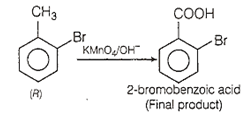
Step 5:On oxidation with KMnO4/ OH-, -CH3 group changes to -COOH group.
In double strand helix structure of DNA, heterocyclic base cytosine forms hydrogen bond with
Adenine
Guanine
Purine
Uracil
0ne mole of hydrazine (N2H4) loses 10 moles of electrons in a reaction to form a new compound X. Assuming that all the nitrogen atoms in hydrazine appear in the new compound, what is the oxidation state of nitrogen in X? (Note - There is no change in the oxidation state of hydrogen in the reaction).
-1
-3
+3
+5
