 Multiple Choice Questions
Multiple Choice QuestionsWhich of the following metal ions has a calculated magnetic moment value of ✓24. BM?
Mn2+
Fe2+
Fe3+
Co2+
Xenon reacts with fluorine at 873 Kand 7 bar to form XeF4. It his reaction, the ratio of xenon and fluorine required is
1:5
10:1
1:3
5:1
The packing efficiency of simple cubic (sc), body centred cubic (bcc) and cubic close packing (ccp) lattices follow the order
bcc <ccp <sc
ccp <bcc <sc
sc <ccp <bcc
sc <bcc <ccp
The experimental depression in freezing point of a dilute solution is 0.025 K. If the van't Hoff factor (i) is 2.0, the calculated depression in freezing point (in K) is
0.00125
0.025
0.0125
0.05
The molality of an aqueous dilute solution containing non-volatile solute is 0.1 m. What is the boiling temperature (in °C) of solution? (Boiling point elevation constant, Kb = 0.52 kg mol-1K; boiling temperature of water = 100°C).
100.0052
100.052
100.0
100.52
Which one of the following is correct plot of Λm ( in S cm2 mol-1) and √C (in mol/L1/2) for KCl solution? ( y= Λm; x= √C)



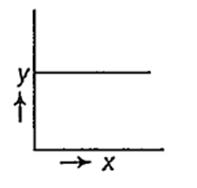
B.

Molar conductivity is given as
