 Multiple Choice Questions
Multiple Choice QuestionsThe enthalpy of the formation of CO2 and H2O are -395 kJ and -285 kJ respectively and the enthalpy of combustion of acetic acid is 869 kJ. The enthalpy of formation of acetic acid is
340 kJ
420 kJ
491 kJ
235 kJ
Considering entropy(s) as a thermodynamic parameter, the criterion for the spontaneity of any process, the change in entropy is
C.
According to law of entropy (also known as second law of thermodynamics), the entropy for a spontaneity of any process is always accompained by an increase in total entropy of universe, i.e.
At low pressure and high temperature, the van der Waal's equation is finally reduced (simplified) to
pVm = RT
Which of the following electron has minimum energy?
n = 4; l = 0; m= 0; s = +
n= 4; l= 1; m = ±1; s = +
n = 5; l = 0; m = 0; s = +
n = 3; l = 2; m = -2; s = +
The increasing order of the first ionisation enthalpies of the elements B, P, S and F is
B < S < P < F
F < S < P < B
P < S < B < F
B < P < S < F
Rank the following in decreasing order of basic strength
(i) CH3-CH2-C≡C-
(ii) CH3-CH2-S-
(iii) CH3-CH2-CO
(iv) CH3-CH2-O-
(iv) > (i) > (ii) > (iii)
(i) > (iv) > (ii) > (iii)
(i) > (iv) > (iii) > (ii)
(ii) > (i) > (iv) > (iii)
Which of the following molecules is optically active?
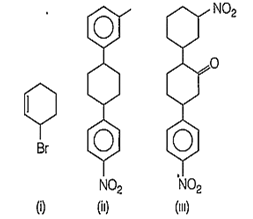
(i) and (iii)
(ii) and (iii)
(i), (ii) and (iii)
(i) and (ii)
Provide the systematic name of the compound shown
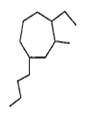
4- butyl - 2-ethyl - 1- methylcycloheptane
1- butyl - 4-ethyl - 3- methylcycloheptane
2- butyl - 4-ethyl - 1- methylcycloheptane
4- butyl - 1-ethyl - 2- methylcycloheptane
