 Multiple Choice Questions
Multiple Choice QuestionsThe reagent commonly used to determine hardness of water titrimetrically is
oxalic acid
disodium salt of EDTA
sodium citrate
sodium thiosulphate
On dissolving moderate amount of sodium metal in liquid NH3 at low temperature, which one of the following does not occur?
Blue coloured solution is obtained.
Na+ ions are formed in the solution.
Liquid NH3 becomes good conductor of electricity
Liquid ammonia remains diamagnetic
D.
Liquid ammonia remains diamagnetic
When moderate amount of sodium metal in liquid NH3 is dissolved, liquid ammonia remains dimagnetic. This is because the alkali metals dissolve in liquid ammonia without evolution of hydrogen. The colour of the dilute solution is blue and are paramagnetic in nature.
M → M+ (in liquid ammonia) + e- (ammoniated)
M + (x + y) NH3 → [M(NH3)x]+ + e- (NH3)y
IUPAC name of the following compound is
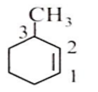
3-methyl cyclohexane
1-methyl cyclohex-2-ene
6-methyl cyclohexene
1-methyl cyclohex-5-ene
The treatment of benzene with isobutene in the presence of sulphuric acid gives
iso -butyl benzene
tert -butyl benzene
n -butyl benzene
no reaction
Among the following, the achiral amino acid is
2-ethylalanine
2-methylglycine
2-hydroxymethyl serine
tryptophan
