 Multiple Choice Questions
Multiple Choice QuestionsFor the reaction of one mole of zinc dust with one mole of sulphuric acid in a bomb calorimeter, U and w corresponds to
U < 0, w = 0
U < 0, w < 0
U > 0, w = 0
U > 0, w > 0
For reaction, 2NOCl (g) 2NO (g) + Cl2 (g), Kc at 427°C is 3 × 10-6 L mol-1. The value of Kp is nearly
7.50 × 10-5
2.50 × 10-5
2.50 × 10-4
1.75 × 10-4
For the chemical equilibrium,
CaCO3(s) CaO(s) + CO2(g)
can be determined from which one of the following plots?
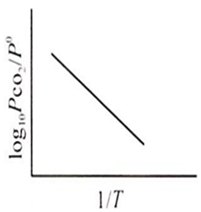
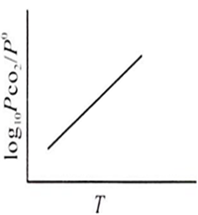
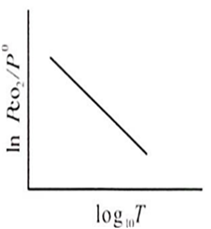
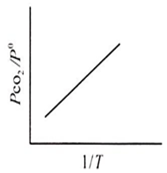
Assertion : Potassium ferrocyanide is diamagnetic whereas potassium ferricyanide is paramagnetic.
Reason : Crystal field splitting in ferrocyanide ion is greater than that of ferricyanide ion.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
Assertion : SeCl4 does not have a tetrahedral structure.
Reason : Se in SeCl4 has two lone pairs.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
C.
If assertion is true but reason is false.
SeCl4 posses see saw geometry, which can be regarded as a distorted trigonal bipyramidal structure, having one lone pair (lp) of electrons in the basal position of the trigonal bi-pyramidal. It arises due to sp3d hybridisation of the central atom. The distortion is basically due to the presence of one lone pair of electrons.
Assertion : First ionization energy for nitrogen is lower than oxygen.
Reason : Across a period effective nuclear charge decreases.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
Assertion : B2 molecule is diamagnetic.
Reason : The highest occupied molecular orbital is of σ type.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
In the balanced chemical reaction
IO3- + aI- + bH+ → cH2O + dI2
a, b, c and d respectively corresponds to
5, 6, 3, 3
5, 3, 6, 3
3, 5, 3, 6
5, 6, 5, 5
Among the following pairs of ions, the lower oxidation state in aqueous solution is more stable than the other, in
Tl+, Tl3+
Cu+, Cu2+
Cr2+, Cr3+
V2+, VO2+
