 Multiple Choice Questions
Multiple Choice QuestionsIdentify the correct statement for the change of Gibbs energy for a system (ΔGsystem) at constant temperature and pressure
If ΔGsystem > 0, the process is spontaneous
If ΔGsystem =0, the system has attained equilibrium
If ΔGsystem < 0, the system is still moving in a particular direction
If ΔGsystem < 0, the system is still moving in a particular direction
Assume each reaction is carried out in an open container. For which reaction will ΔH = ΔE?
H2 (g) + Br2 (g) →2HBr (g)
C (s) + 2 H2O (g) → 2 H2 (g) + CO2 (g)
PCl5 (g) →PCl3 (g) + Cl2 (g)
PCl5 (g) →PCl3 (g) + Cl2 (g)
Given: The mass of electron is 9.11 x 10-31 kg
Planck constant is 6.626 x 10-34 Js,
the uncertainty involved in the measurement of velocity within a distance of 0.1 A is:
5.79 x 106 ms-1
5.79 x 107 ms-1
5.79 x 108 ms-1
5.79 x 108 ms-1
The enthalpy and entropy change for the reaction:
Br2 (l) + Cl2 (g)→ 2BrCl (g)
are 30 kJ mol-1 and 105 JK-1 mol-1 respectively. The temperature at which the reaction will be in equilibrium is:
285.7 K
273 K
450 K
450 K
For the reaction,
CH4 (g) + 2 O2 (g) ⇌ CO2 (g) + 2H2O (l),
ΔrH = - 170. 8 kJ mol-1
Which of the following statements is not true?
At equilibrium, the concentrations of CO2 (g) and H2O (l) are not equal
The equilibrium constant for the reaction is given by Kp = 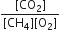
Addition of CH4 (g) or O2 (g) at equilibrium will cause a shift to the right
Addition of CH4 (g) or O2 (g) at equilibrium will cause a shift to the right
The enthalpy of combustion of H2, cyclohexene (C6H10) and cyclohexene (C6H12) are -241, -3800 and -3920 kJ per mol respectively.The heat of hydrogenation of cyclohexane is:
-212 kJ mol
+121 kJ mol
+242 kJ per mol
+242 kJ per mol
A.
-212 kJ mol
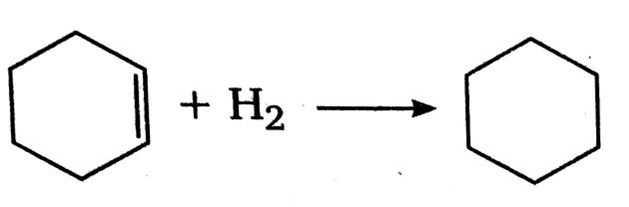
ΔH= [ΔH of combustion of cyclohexane -(ΔH of combustion of cyclohexene +ΔH of combustion of H2)]
= -[-3920 -(3800-24)] kJ
= - [3920 + 4041] kJ
=-[121] kJ
=--121 kJ
Which of the following pairs consitutes a buffer?
HNO2 and NaNO2
NaOH and NaCl
HNO3 and NH4NO3
HNO3 and NH4NO3
The hydrogen ion concentration of a 10-8 M HCL aqueous solution at 298 K (Kw = 10-14) is:
1.0 x 10-6
1.0525 x 10-7 M
9.525 x 10-8 M
9.525 x 10-8 M
The orientation of an atomic orbital is governed by:
azimuthal quantum number
spin quantum number
magnetic quantum number
magnetic quantum number
