 Multiple Choice Questions
Multiple Choice QuestionsNitrobenzene on treatment with zinc dust and aqueous ammonium chloride gives
C6H5N =N-C6H5
C6H5NH2
C6H5NO
C6H5NHOH.
Methyl--glucoside and methyl--D-glucoside are
epimers
anomers
enantiomers
conformational diastereomers.
Which ofthe following compounds has the highest boiling point?
CH3CH2CH2Cl
CH3CH2CH2CH2Cl
CH3CH(CH3)CH2Cl
(CH3)3CCl
The correct increasing order of the reactivity of halides for SN 1 reaction is
CH3- CH2- X<(CH3)2CH= X <CH2= CH-CH2- X< PhCH2- X
(CH3)2CH-X <CH3-CH2- X < CH2= CH - CH2-X < PhCH2-X
PhCH2- X<(CH3)2CH=X<CH3-CH2-X<CH2= CH- CH2- X
CH2= CH - CH2- X < Ph- CH2- X <(CH3)2CH-X <CH3-CH2- X
Assertion : In the iodometric titration starch is used as an indicator.
Reason : Starch is a polysaccharide.
If both assertion and reason are true and reason is the correct explanation of the assertion.
If both assertion and reason are true but reason is not the correct explanation of the assertion.
If assertion is true, but reason is false.
Both assertion and reason are false statements.
Assertion: Anilinium chloride is more acidic than ammonium chloride.
Reason : Anilinium ion is resonance-stabilised.
If both assertion and reason are true and reason is the correct explanation of the assertion.
If both assertion and reason are true but reason is not the correct explanation of the assertion.
If assertion is true, but reason is false.
Both assertion and reason are false statements.
C.
If assertion is true, but reason is false.
D.
Both assertion and reason are false statements.
Aniline is weaker base than ammonium chloride. In NH4Cl or aliphatic amines. the non-bonding electron pair of N is localized and is fully available for coordination with a proton. On the other hand, in aniline or other aromatic amines, the non-bonding electron pair is delocalisecl into benzene ring by
resonance.
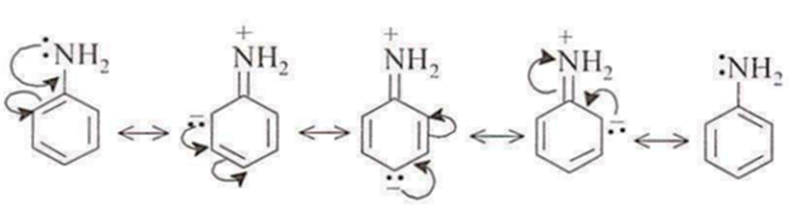
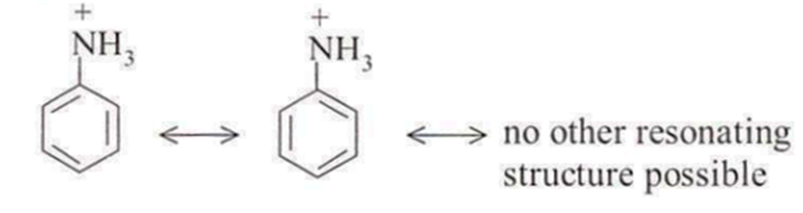
But anilinium ion is less resonance stabilised than aniline.
Assertion : 1,3-butadiene is the monomer for natural rubber.
Reason : Natural rubber is formed through anionic addition polymerization.
If both assertion and reason are true and reason is the correct explanation of the assertion.
If both assertion and reason are true but reason is not the correct explanation of the assertion.
If assertion is true, but reason is false.
Both assertion and reason are false statements
