 Multiple Choice Questions
Multiple Choice QuestionsA Weak acid, HA has a Ka of 1.00 x 10-5. If 0.100 mole of this percentage of acid dissociated at equilibrium is closest to:
99.0%
1.00%
99.9 %
99.9 %
Which of the following oxidation states are the most characteristics for lead and tin respectively?
+4,+2
+2,+4
+4,+4
+4,+4
The correct order of C-O bond length among CO, CO32-, CO2 is,
CO2< CO32-< CO
CO < CO32-< CO2
CO32- <CO2 <CO
CO32- <CO2 <CO
The following equilibrium constants are given:
N2 + 3H2 ⇌ 2NH3; K1
N2 +O2 ⇌ 2NO; K2
H2 + 1/2O2 ⇌ H2O' K3
The equilibrium constants for the oxidation of NH3 by oxygen to give NO is:
K2K33 /K1
KK2K32 /K1
K22K3 /K1
K22K3 /K1
Consider the following sets of quantum numbers:
| n | l | m | s | |
| i) | 3 | 0 | 0 | +1/2 |
| ii) | 2 | 2 | 1 | +1/2 |
| iii) | 4 | 3 | -2 | -1/2 |
| iv) | 1 | 0 | -1 | -1/2 |
| v) | 3 | 2 | 3 | +1/2 |
ii, iii and iv
i, ii, iii and iv
ii, iv and v
ii, iv and v
Consider the following reactions: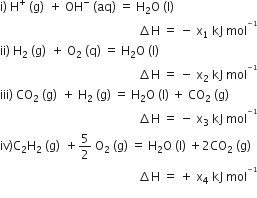
Enthalpy of formation of H2O (l) is:
- x2 kJ mol-1
+ x3 kJ mol-1
- x4 kJ mol-1
- x4 kJ mol-1
Given that bond energy of H-H and Cl- Cl are 430 kJ mol-1 and 240 kJ mol-1 respectively and ΔHf for HCl is -90 kJ mol-1. Bond and ΔHf for HCl is -90 kJ mol-1.Bond enthalpy of HCl is:
290 kJ mol-1
380 kJ mol-1
425 kJ mol-1
425 kJ mol-1
With which of the following electronic configuration an atom has the lowest ionisation enthalpy?
1s2 2s22p5
1s2 2s22p3
1s2 2s22p5 3s1
1s2 2s22p5 3s1
An element, X has the following isotopic composition;
200X: 90%
199X : 8.0%
202X ; 2.0 %
The weighted average atomic mass of the naturally -occurring element X is closet to:
200 amu
201 amu
202 amu
202 amu