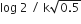Multiple Choice Questions
Multiple Choice QuestionsWhich one of the following ionic species has the greatest proton affinity to form stable compound?
HS-
NH2-
F-
F-
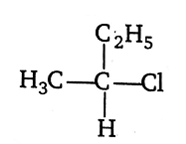
CH3-CHCl-CH2-CH3 has a chiral centre. Which one of the following represents its R configuration?



0.5 Molal aqueous solution of aweak acid (HX) is 20% ionised. If Kf for water is1.86 K kg mol-1, the lowering in freezing point of the solution is:
-1.12 K
0.56 K
1.12 K
1.12 K
The Langmuir adsorption isotherm is deduced using the assumption:
the adsorption takes place in multilayers
The adsorption sites are equivalent in their ability to adsorb the particles
The adsorbed molecules interact with each other
The adsorbed molecules interact with each other
A.
the adsorption takes place in multilayers
The main points of Langmuir theroy of adsorption are as:
i) Adsorption takes place on the surface of the solid only till the whole of the surface is completely covered with a unimolecular layer of the adsorbed gas.
ii) Adsorption consist fo two opposing processes (a) condensation, and (b) evaporation.
iii) The rate of condensation depend upon the uncovered surface of the adsorbent available for condensation.
The reaction of hydrogen and iodine monochloride is given as:
H2 (g) + 2ICl (g) → 2 HCl (g) + I2 (g)
This reaction is of first order with respect to H2 (g) and ICI (g), following mechanisms were proposed:
Mechanism A:
H2 (g) + 2 ICl (g) → 2 HCl (g) + I2 (g)
Mechanism B:
H2 (g) + ICl (g) →HCl (g) + HI (g) ;slow
HI (g) + ICl (g) → HCl (g) + I2 (g); fast
Which of the above mechanism (s) can be consistent with the given information about the reaction?
B only
A and B both
Neither A nor B
Neither A nor B
Which one of the following anions is present in the chain structure silicates ?
Si2O76-
(Si2O56-)n
(SiO32-)n
(SiO32-)n
For the following:
(i) I- (ii) Cl- (iii) Br-
the increasing order of nucleophilicity would be:
I- < Br- < Cl-
Cl- < Br- < I-
I- < Cl- < Br-
I- < Cl- < Br-
Which one of the following orders correctly represents the increasing acid strengths of the given acids?
HOCl < HOClO < HOClO2 < HOClO3
HOClO < HOCl < HOClO3 < HOClO2
HOClO2 < HOClO3 < HOClO < HOCl
HOClO2 < HOClO3 < HOClO < HOCl
In a first order reaction A →B, if k israte constant and initial concentration of the reactant A is 0.5 A M then the half -life is :
0.693/0.5 k
log2/k