 Multiple Choice Questions
Multiple Choice QuestionsNames of some compounds are given. Which one is not correct in IUPAC system?
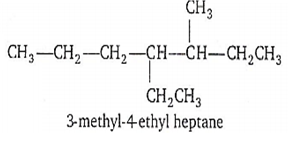
D.


Correct IUPAC name is 4-ethyl-3-methyl heptane because substituents are written in alphabetical order.
The best method for the separation of naphthalene and benzoic acid from their mixture is
chromatography
crystallisation
distillation
sublimation
At 25°C, the dissociation constant of a base, BOH, is 1.0 x 10-12. The concentration of
hydroxyl ions in 0.01 M aqueous solution of the base would be
2.0 x 10-6 mo L-1
1.0 x 10-5 mo L-1
1.0 x 10-6 mo L-1
1.0 x 10-7 mo L-1
The aqueous solution containing which one of the following ions will be colourless ? (Atomic no. Sc= 21, Fe= 26, Ti= 22, Mn = 25)
Sc3+
Fe2+
Ti3+
Mn2+
What is the correct relationship between the pHs of isomolar solutions of sodium oxide (pH1), sodium sulphide (pH2), sodium selenide (pH3) and sodium telluride (pH4)?
pH1 > pH2 pH3 > pH4
pH1 < pH2 pH3 < pH4
pH1 < pH2 pH3 pH4
pH1 > pH2 pH3 > pH4
For a first order reaction A B, the reaction rate at reactant concentration of 0.01 M is found to be 2.0 x 10-5 mol L-1s-1. The half-life period of the reaction is
220 s
30 s
300 s
347 s
Which one of the following forms micelles in aqueous solution above certain concentration?
Urea
Dodecyl trimethyl ammonium chloride
Pyridinium chloride
Glucose
The rate of reaction between two reactants A and B decreases by a factor of 4, if the concentration of reactant B is doubled. The order of this reaction with respect to reactant B is
-1
-2
1
2
A solution of urea (mol. mass 56g mol-1) boils at 100.18°C at the atmospheric pressure. If kf and kb for water are 1.86 and 0.512 K kg mol-1 respectively, the above solution will freeze at
-6.54°C
6.54°C
0.654°C
- 0.654°C
