 Multiple Choice Questions
Multiple Choice QuestionsThe pH of the solution obtained on neutralisation of 40 mL 0.1 M NaOH with 40 mL 0.1 M CH3COOH is
7
8
6
3
Inert gases are mixed in iodine vapours. Then there are _____ between them.
H-bonding
van der Waals forces
electrostatic forces
metallic bonds
In P versus V graph, the horizontal line is found in which exists
Gas
Liquid
Equilibrium between gas and liquid
Super critical temperature
During estimation of nickel, we prepare nickel dimethylglyoxime, a scarlet red solid. This compound is
ionic
covalent
metallic
non-ionic complex
Critical temperatures for A, B, C and D gases are 25°C, 10°C, -80°C and 15°C respectively. Which gas will be liquefied more easily?
A
B
C
D
During titration of acetic acid with aqueous NaOH solution, the neutralisation graph has a vertical line. This line indicates
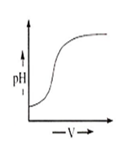
alkaline nature of equivalence
acidic nature of equivalence
neutral nature of equivalence
depends on experimental proceeding
A.
alkaline nature of equivalence
In any type of acid-base titration, there is a sudden change in the pH value at the end point. During the titration of a weak acid with a strong base, beyond the equivalent point, the solution will contain the salt and excess of free base.
CH3COOH + NaOH → + H2O
Hence, due to the presence of free base, solution becomes alkaline in nature.
Calculate change in internal energy if H = - 92.2 kJ, P = 40 atm and V = -1L.
-42 kJ
-88 kJ
+88 kJ
+42 kJ
