 Multiple Choice Questions
Multiple Choice QuestionsBase strength of 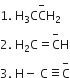
is in the order of
(2) > (1) > (3)
(3) > (2) > (1)
(1) > (3)> (2)
(1) > (3)> (2)
What volume of oxygen gas (O2) measured at 0oC and 1 atm, is needed to burn completely 1 L of propane gas (C3H8) measured under the same conditions?
7 L
6 L
5 L
5 L
Equimolar solutions of the following were prepared in water separately which one of the solutions will record the highest pH?
SrCl2
BaCl2
MgCl2
MgCl2
The measurment of the electron position is associated with an uncerrtanity in momentum, which equal to 1 x 10-18 g cm s-1.The uncertanity in electron velocity is,
(mass of an electrons is 9 x 10-28 g)
1 x 108
1 x 106 cm s-1
1 x 105 cm s-1
1 x 105 cm s-1
The correct order of increasing bond angles in the following triatomic species is




B.

As the number of lone pair of electrons increases, bond angle decreases. NO2+ ion is isoelectronic with the CO2 molecule. It is a linear ion and its central atom (N+) undergoes sp-hybridisation, hence bond angle is 180o.
In NO2- ion, N -atom undergoes sp2 hybridisation. The angle between hybrid orbital should be 120o but one lone pair of electrons is lying on N- atom, hence bond angle decreases to 115o.
In NO2 molecule, N -atom has one unpaired electron in a sp2-hybrid orbital. The bond angle should be 120o but actually, it is 132o. It may be due to one unpaired electron in a sp2-hybrid orbital.
Therefore, the increasing order of bond angles.
Four diatomic solutions of the following were prepared in water separately. Which one of the solutions will record the highest pH?
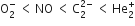
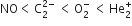
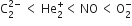
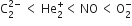
An Organic compound contains carbon, hydrogen and oxygen. Its elemental analysis gave C, 38.71% and H, 9.67%. The empirical formula of the compound would be
CH3O
CH2O
CHO
CHO
How many moles of lead (II) chloride will be formed from a reaction between 6.5 g of PbO and 3.2 g of HCl?
0.044
0.333
0.011
0.011
Bond dissociation enthalpy of H2, Cl2 and HCl and 434, 242 and 431 kJ mol-1 respectively. Enthalpy of formation of HCl is
93 kJ mol-1
-245 kJ mol-1
-39 kJ mol-1
-39 kJ mol-1
The dissociation equilibrium of gas AB2 can be represented as
2AB2 (g) ⇌ 2AB (g) + B2 (g)
The degree of dissociation is 'x' and is small compared to 1. The expression relating the degree of dissociation (x) with equilibrium constant Kp and total pressure p is
(2Kp / p)
(2Kp/p)1/3
(2Kp/p)1/2
(2Kp/p)1/2
