 Multiple Choice Questions
Multiple Choice QuestionsWhich one of the following arrangement does not give the correct picture of the trends indicated against it?
F2 > Cl2 > Br2 > I2 : Oxidising power
F2 > Cl2 > Br2 > I2 : Electron gain enthalpy
F2 > Cl2 > Br2 > I2 : Bond dissociation energy
F2 > Cl2 > Br2 > I2 : Bond dissociation energy
Standard free energies of formation (in kJ/mol) at 298 K are -237.2, 394.4 and -8.2 for H2O (l), CO2 (g) and pentane (g), respectively. The value of Ecello for pentane-oxygen fuel cell is
1.968 V
2.0968 V
1.0968 V
1.0968 V
Which of the following statements is not correct?
The fraction of the total volume occupied by the atoms in a primitive cell is 0.48
molecules solids are generally volatile
the number of carbon atoms in a unit cell
the number of carbon atoms in a unit cell
If 'a' stands for the edge length of the cubic system:simple cubic , body centred cubic and face centred cubic, then the ratio of radii of the spheres in these systems will be repsectively,
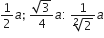

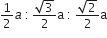
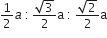
A.
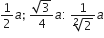
For simple cubic,
a = 2r
r = a/2
For body centred cubic,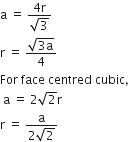
Hence, the ratio of radii in simple cubic, body centred cubic and face centred cubic is 
The rate constant k1 and k2 for two different reactions are 1016. e-2000/T and 1015.e-1000/T respectively. The temperature at which k1 = k2
1000 K
2000/2.303 K
2000 K
2000 K
With which one of the following elements silicon should be doped so as to give p - type of semiconductor?
Germanium
Arsenic
Selenium
Selenium
The bromination of acetone cytosine and guanine solution is represented by this equation.
CH3COCH3 (aq) + Br2 (aq) →CH3COCH2Br (aq) + H+ (aq) + Br- (aq)
These kinetic data were obtained for given reaction concentrations.
|
Initial Concentrations, M
|
||
| [CH3COOH] | [Br2] | [H+] |
| 0.30 | 0.05 | 0.05 |
| 0.30 | 0.10 | 0.05 |
| 0.30 | 0.10 | 0.10 |
| 0.40 | 0.05 | 0.20 |
Rate = k[CH3COCH3][H+]
Rate = k[CH=COCH3][Br2]
Rate = k [CH3COCH3][Br2][H+]
Rate = k [CH3COCH3][Br2][H+]
In which of the following coordination entities the magnitude of Δo (CFSE in the octahedral field) will be maximum?
(Atomic number Co = 27)
[Co(H2O)6]3+
[CO(NH3)6]3+
[CO(CN)6]3-
[CO(CN)6]3-
