 Multiple Choice Questions
Multiple Choice QuestionsPure silicon doped with phosphorus is a
metallic conductor
insulator
n-type semiconductor
p-type semiconductor
C.
n-type semiconductor
Excess of one valence electron in P atom (5 valence electron) over Si (4 valence electron) will form n-type semiconductor.
IUPAC name of the following compound
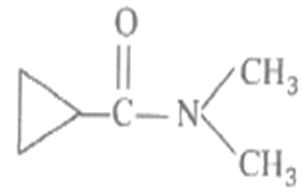
N, N- dimethylcyclopropane carboxamide
N-methylcyclopropanamide
cyclopropionamide
None of the above
Sodium formate on heating gives
oxalic acid and H2
sodium oxalate and H2
sodium oxalate
CO2 and caustic soda
Which does not give a precipitate with AgNO3 solution?
[Co(NH3)6]Cl3
[Co(NH3)5Cl]Cl2
[Co(NH3)4Cl2]Cl
[Co(NH3)3Cl3]
Total volume of atoms present in a face centred cubic unit cell of a metal is (r is atomic radius)
Gold number is associated with
amount of gold
protective colloids
purple of cassius
electrophoresis
Noble gases are used in discharge tubes to give different colours. Reddish-orange glow is due to
Ar
Ne
Xe
Kr
