 Multiple Choice Questions
Multiple Choice QuestionsFrom the following bond energies:
H-H bond energy: 431.37 kJ mol-
C=C bond energy: 606.10 kJ mol-
C- C bond energy: 336.49 kJ mol-
C-H bond energy: 410.50 kJ mol-
Enthalpy for the reaction,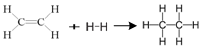
will be
1523.6 kJ mol-
-243.6 kJ mol-
-1200. kJ mol-
-1200. kJ mol-
Which one of the element with the following outer orbital configuration may exhibit the largest number of oxidation states?
3d5,4s2
3d5,4s1
3d5,4s2
3d5,4s2
The ionisation constant of ammonium hydroxide is 1.77 x 10-5 at 298 K. Hydrolysis constant of ammonium chloride is
5.65 x 10-10
6.50 x 10-12
5.65 x 10-13
5.65 x 10-13
Oxidation number of P in PO43-, of S in SO42- and that of Cr in Cr2O72- are respectively,
+5,+6 and +6
+3, + 6 and +5
+5,+3 and +6
+5,+3 and +6
Amongst the elements with following electronic configuration, which one of them may have the highest ionisation energy?
[Ne] 3s2 3p3
[Ne] 3s2 3p2
[Ar] 3d10, 4s2 4p3
[Ar] 3d10, 4s2 4p3
Maximum number of electrons in a subshell of an atom is determined by the following
4l + 2
2l +1
4l-2
4l-2
The values of ΔH and ΔS for the reaction, C(graphite) + CO2(g) → 2 CO (g) are 170 kJ and 170 JK-1 respectively. This reaction will be spontaneous at
710 K
910 K
1110 K
1110 K
Nitrobenzene can be prepared from benzene by using a mixture of conc. HNO3 and Conc. H2SO4.In the mixture, nitric acid acts as a/an:
reducing agent
acid
base
base
According to MO theory which of the following lists ranks the nitrogen species in terms of increasing bond order?



