 Multiple Choice Questions
Multiple Choice QuestionsThe state of hybridization of C2, C3, C5 and C6 of the hydrocarbon,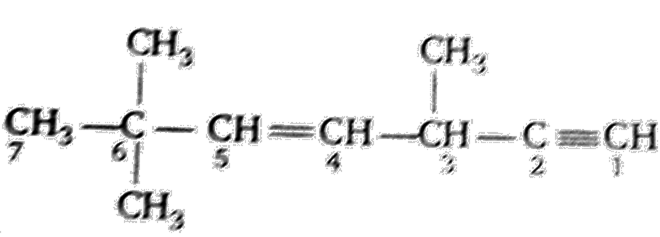
is in the following sequence
sp, sp3, sp2 and sp3
sp3, sp2, sp2 and sp
sp,sp2,sp2 and sp3
sp,sp2,sp2 and sp3
The dissociation constants for acetic acid and HCN at 25o C are 1.5 X 10-5 and 4.5 x 10-10 respectively. The equilibrium constant for the equilibrium,
CN- + CH3COOH and HCN + CH3COO-
would be
3 x 105
3.0 x 10-5
3.0 x 10-4
3.0 x 10-4
In which of the following molecules/ions BF3, NO2-, NH2- and H2O, the central atom is sp2 hybridised?
NO2- and NH2-
NH2- and H2O
NO2- and H2O
NO2- and H2O
D.
NO2- and H2O
For sp2 hybridization, there must be either 3 sigma bonds or two sigma bonds and one lone pair of electrons in the molecules or ions.
In BF3 molecule, a number of sigma bond is 3 ie, sp2 hybridization.
In NO2- molecule, the number of sigma bond is 2 and the number of lone pairs is 2 ie, sp3 hybridization.
In NH2-molecule, the number of sigma bond is 2 and the number of lone pairs is 2 ie, sp3 hybridization.
In H2O molecule, the number of sigma bond is 2 and number of the lone pair are 2 ie, sp3 hybridization.
Thus, in BF3 and NO2- central atom is sp2 hybridised.
10 g of hydrogen and 64 of oxygen were filled in a steel vessel and exploded. Amount of water produced in this reaction will be
2 mol
3 mol
4 mol
4 mol
The energy absorbed by each molecule (A2) of a substance is 4.4 x 10-19 J and bond energy per molecule is 4.0 X 10-19 J. The kinetic energy of the molecule per atom will be
2.0 x 10-20 J
2.2 x 10-19 J
2.0 x 10-19 J
2.0 x 10-19 J
What is the dominant intermolecular force or bond that must be overcome in converting liquid CH3OH to a gas?
Hydrogen bonding
Dipole-dipole interaction
Covalent bonds
Covalent bonds
Which of the following is not permissible arrangement of electrons in an atom?
n = 4, l=0, m=0, s=-1/2
n=5, l=3, m=0, s=+1/2
n=3, l=2, m=-3, s= -1/2
n=3, l=2, m=-3, s= -1/2
The IUPAC name of the compound having the formula 
3-butene-1-yne
1-butyn-3-ene
but-1-yne-3-ene
but-1-yne-3-ene
In the case of alkali metals, the covalent character decreases in the order
MCl> MI>MBr>MF
MF> MCl> MBr>MI
MF > MCl> MI>MBr
MF > MCl> MI>MBr
which of the following oxides is not expected to react with sodium hydroxide?
B2O3
CaO
SiO2
SiO2
